Abstract
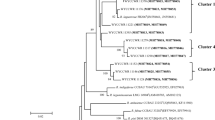
Henan Province is a major area of peanut production in China but the rhizobia nodulating the crop in this region have not been described. A collection of 217 strains of peanut rhizobia was obtained from six field sites across four soil types in Henan Province, North China, by using peanut as a trap host under glasshouse conditions. The 217 strains separated into 8 distinct types on PCR–RFLP analysis of their IGS sequences. Phylogenetic analysis of the 16S rRNA, recA, atpD, and glnII genes of 11 representative strains of the 8 IGS types identified Bradyrhizobium guangdongense, B. ottawaense and three novel Bradyrhizobium genospecies. Bradyrhizobium guangdongense was dominant, accounting for 75.0% of the total isolates across the field sites while B. ottawaense covered 5.1% and the three novel Bradyrhizobium genospecies 4.1 to 8.8% of the total. The symbiosis-related nodA and nifH gene sequences were not congruent with the core genes on phylogenetic analysis and separated into three groups, two of which were similar to sequences of Bradyrhizobium spp. isolated from peanut in south-east China and the third identical to that of B. yuanmingense isolated from Lespedeza cuneata in northern China. A canonical correlation analysis between the distribution of IGS genotypes and soil physicochemical characteristics and climatic factors indicated that the occurrence of IGS types/species was mainly associated with soil pH and available phosphorus.



Similar content being viewed by others
References
Hammons RO (1994) The origin and history of the groundnut. In: Smartt J (ed) The groundnut crop. Springer, Netherlands, pp 24–42. https://doi.org/10.1007/978-94-011-0733-4_2
Seijo G, Lavia GI, Fernandez A, Krapovickas A, Ducasse DA, Bertioli DJ, Moscone EA (2007) Genomic relationships between the cultivated peanut (Arachis hypogaea, Leguminosae) and its close relatives revealed by double GISH. Am J Bot 94:1963–1971. https://doi.org/10.3732/ajb.94.12.1963
Okito A, Alves BJR, Urquiaga S, Boddey RM (2004) Nitrogen fixation by groundnut and velvet bean and residual benefit to a subsequent maize crop. Pesqui Agropecu Bras 39:1183–1190. https://doi.org/10.1590/s0100-204x2004001200004
Toomsan B, Mcdonagh JF, Limpinuntana V, Giller KE (1995) Nitrogen fixation by groundnut and soyabean and residual nitrogen benefits to rice in farmers’ fields in Northeast Thailand. Plant Soil 175:45–56. https://doi.org/10.1007/BF02413009
Andrews M, Andrews ME (2017) Specificity in legume rhizobia symbioses. Int J Mol Sci 18:705
Dos Santos JWM, da Silva JF, Ferreira TDD, Dias MAM, Fraiz ACR, Escobar IEC, dos Santos RC, de Lima LM, Morgante CV, Fernandes PI (2017) Molecular and symbiotic characterization of peanut bradyrhizobia from the semi-arid region of Brazil. Appl Soil Ecol 121:177–184. https://doi.org/10.1016/j.apsoil.2017.09.033
Zazou AZ, Fonceka D, Fall S, Fabra A, Ibanez F, Pignoly S, Diouf A, Toure O, Faye MN, Hocher V, Diouf D, Svistoonoff S (2018) Genetic diversity and symbiotic efficiency of rhizobial strains isolated from nodules of peanut (Arachis hypogaea L.) in Senegal. Agric Ecosyst Environ 265:384–391
FAOSTAT (2021) Food and Agriculture Organisation of the United Nations. www.fao.org/faostat. Accessed 17 May 2021
Yang JK, Zhou JC (2008) Diversity, phylogeny and host specificity of soybean and peanut bradyrhizobia. Biol Fertil Soils 44:843–851. https://doi.org/10.1007/s00374-008-0269-3
Chen J, Hu M, Ma H, Wang Y, Wang ET, Zhou Z, Gu J (2016) Genetic diversity and distribution of bradyrhizobia nodulating peanut in acid-neutral soils in Guangdong Province. Syst Appl Microbiol 39:418–427. https://doi.org/10.1016/j.syapm.2016.06.002
Shao S, Chen M, Liu W, Hu X, Li Y (2020) Long-term monoculture reduces the symbiotic rhizobial biodiversity of peanut. Syst Appl Microbiol 43:126101. https://doi.org/10.1016/j.syapm.2020.126101
Chang YL, Wang JY, Wang ET, Liu HC, Sui XH, Chen WX (2011) Bradyrhizobium lablabi sp. nov., isolated from effective nodules of Lablab purpureus and Arachis hypogaea. Int J Syst Evol Microbiol 61:2496–2502. https://doi.org/10.1099/ijs.0.027110-0
Wang R, Chang YL, Zheng WT, Zhang D, Zhang XX, Sui XH, Wang ET, Hu JQ, Zhang LY, Chen WX (2013) Bradyrhizobium arachidis sp. nov., isolated from effective nodules Arachis hypogaea grown in China. Syst Appl Microbiol 36:101–105. https://doi.org/10.1016/j.syapm.2012.10.009
Li YH, Wang R, Zhang XX, Young JPW, Wang ET, Sui XH, Chen WX (2015) Bradyrhizobium guangdongense sp. nov. and Bradyrhizobium guangxiense sp. nov., isolated from effective nodules of peanut. Int J Syst Evol Microbiol 65:4655–4661. https://doi.org/10.1099/ijsem.0.000629
Li YH, Wang R, Sui XH, Wang ET, Zhang XX, Tian CF, Chen WF, Chen WX (2019) Bradyrhizobium nanningense sp. nov., Bradyrhizobium guangzhouense sp. nov. and Bradyrhizobium zhanjiangense sp. nov., isolated from effective nodules of peanut in Southeast China. Syst Appl Microbiol 42:11. https://doi.org/10.1016/j.syapm.2019.126002
Liao BS (2020) A review on progress and prospects of peanut industry in China. Chin J Oil Crop Sci 42:161–166. https://doi.org/10.19802/j.issn.1007-9084.2020115
Zhang JJ, Lou K, Jin X, Mao PH, Wang ET, Tian CF, Sui XH, Chen WF, Chen WX (2012) Distinctive Mesorhizobium populations associated with Cicer arietinum L. in alkaline soils of Xinjiang, China. Plant Soil 353:123–134. https://doi.org/10.1007/s11104-011-1014-5
Han LL, Wang ET, Han TX, Liu J, Sui XH, Chen WF, Chen WX (2009) Unique community structure and biogeography of soybean rhizobia in the saline-alkaline soils of Xinjiang, China. Plant Soil 324:291–305. https://doi.org/10.1007/s11104-009-9956-6
Zhang JJ, Shang YM, Wang ET, Chen WF, de Lajudie P, Li BY, Guo C, Yang X, Zheng JQ, Liu CZ (2018) Mesorhizobium jarvisii sv. astragali as predominant microsymbiont for Astragalus sinicus L. in acidic soils, Xinyang, China. Plant Soil 433:201–212. https://doi.org/10.1007/s11104-018-3830-3
Terefework Z, Kaijalainen S, Lindstrom K (2001) AFLP fingerprinting as a tool to study the genetic diversity of Rhizobium galegae isolated from Galega orientalis and Galega officinalis. J Biotechnol 91:169–180. https://doi.org/10.1016/s0168-1656(01)00338-8
Laguerre G, Mavingui P, Allard MR, Charnay MP, Louvrier P, Mazurier SI, Rigottier-Gois L, Amarger N (1996) Typing of rhizobia by PCR DNA fingerprinting and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions: application to Rhizobium leguminosarum and its different biovars. Appl Environ Microbiol 62:2029–2036. https://doi.org/10.1128/aem.62.6.2029-2036.1996
Laguerre G, Allard MR, Revoy F, Amarger N (1994) Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes. Appl Environ Microbiol 60:56–63. https://doi.org/10.1128/aem.60.1.56-63.1994
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics (Oxford, England) 14:817–818. https://doi.org/10.1093/bioinformatics/14.9.817
Vinuesa P, Silva C, Lorite MJ, Izaguirre-Mayoral ML, Bedmar EJ, Martinez-Romero E (2005) Molecular systematics of rhizobia based on maximum likelihood and Bayesian phylogenies inferred from rrs, atpD, recA and nifH sequences, and their use in the classification of Sesbania microsymbionts from Venezuelan wetlands. Syst Appl Microbiol 28:702–716. https://doi.org/10.1016/j.syapm.2005.05.007
Vinuesa P, Silva C, Werner D, Martinez-Romero E (2005) Population genetics and phylogenetic inference in bacterial molecular systematics: the roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol Phylogenet Evol 34:29–54. https://doi.org/10.1016/j.ympev.2004.08.020
Haukka K, Lindstrom K, Young JP (1998) Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Appl Environ Microbiol 64:419–426. https://doi.org/10.2307/1140518
Laguerre G, Nour SM, Macheret V, Sanjuan J, Amarger N (2001) Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiol 147:981–993. https://doi.org/10.1099/00221287-147-4-981
Lepš J, Šmilauer P (2003) Multivariate Analysis of Ecological Data Using CANOCO 5. Cambridge University Press. https://doi.org/10.1017/CBO9780511615146
Yu X, Cloutier S, Tambong JT, Bromfield ESP (2014) Bradyrhizobium ottowaense sp. nov., a symbiotic nitrogen fixing bacterium from root nodules of soybeans in Canada. Int J Syst Evol Microbiol 64:3202–3207
Yao ZY, Kan FL, Wang ET, Wei GH, Chen WX (2002) Characterisation of rhizobia that nodulate legume species of the genus Lespedeza and description of Bradyrhizobium yuanmingense sp. nov. Int J Syst Evol Microbiol 52:2219–2230
De Lajudie PM, Andrews M, Ardley J, Eardly B, Jumas-Bilak E, Kuzmanović N, Lassalle F, Lindström K, Mhamdi R, Martínez-Romero E, Moulin L, Mousavi SA, Nesme X, Peix A, Pulawska J, Steenkamp E, Stępkowski T, Tian C-F, Vinuesa P, Wei G, Willems A, Zilli J, Young P (2019) Minimal standards for the description of new genera and species of rhizobia and agrobacteria. Int J Syst Evol Microbiol 69:1852–1863
Andrews M, De Meyer S, James EK, Stepkowski T, Hodge S, Simon MF, Young JPW (2018) Horizontal transfer of symbiosis genes within and between rhizobial genera: occurrence and importance. Genes 9:321
Funding
This work was financed by the Project for Extramural Scientists of State Key Laboratory of Agrobiotechnology (Project No. 2021SKLAB6-8) from JJ Zhang and project of Sabbatical Year SIP20200726 authorized by IPN, Mexico from ET Wang.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Peng, S., Li, S. et al. Arachis hypogaea L. from Acid Soils of Nanyang (China) Is Frequently Associated with Bradyrhizobium guangdongense and Occasionally with Bradyrhizobium ottawaense or Three Bradyrhizobium Genospecies. Microb Ecol 84, 556–564 (2022). https://doi.org/10.1007/s00248-021-01852-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-021-01852-2