Abstract
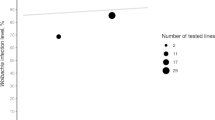
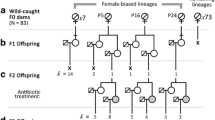
Spiroplasma endosymbionts are maternally transmitted bacteria that may kill infected sons resulting in the production of female-biased broods. The prevalence of male killers varies considerably both between and within species. Here, we evaluate the spatial and temporal status of male-killing and non-male-killing Spiroplasma infection in three Brazilian populations of Drosophila melanogaster, nearly a decade after the first occurrence report for this species. The incidence of the male-killing Spiroplasma ranged from close to 0 to 17.7 % (so far the highest estimate for a Drosophila species) with a suggestion of temporal decline in a population. We also found non-male-killing Spiroplasma coexisting in one population at lower prevalence (3–5 %), and we did not detect it in the other two. This may be taken as a suggestion of a spreading advantage conferred by the male-killing strategy. Sequencing two loci, we identified the phylogenetic position of Spiroplasma strains from the three localities, showing that all strains group closely in the poulsonii clade. Due to intensive sampling effort, we were able to test the association between Spiroplasma infections and another widespread endosymbiont, Wolbachia, whose prevalence ranged from 81.8 to 100 %. The prevalence of Wolbachia did not differ between Spiroplasma-infected and uninfected strains in our largest sample nor were the prevalences of the two endosymbionts associated across localities.


Similar content being viewed by others
References
Anbutsu H, Goto S, Fukatsu T (2008) High and low temperatures differently affect infection density and vertical transmission of male-killing Spiroplasma symbionts in Drosophila hosts. Appl Environ Microbiol 74:6053–6059
Bandi C, Dunn AM, Hurst GDD, Rigaud T (2001) Inherited microorganisms, sex-specific virulence and reproductive parasitism. Trends Parasitol 17:88–94
Cavalcanti AGL, Falcão DN, Castro LE (1957) “Sex-Ratio” in Drosophila prosaltans—a character due to interaction between nuclear genes and cytoplasmic factors. Am Nat 91:327–329
de Vienne DM, Giraud T, Martin OC (2007) A congruence index for testing topological similarity between trees. Bioinformatics 23:3119–3124
Dyer KA, Jaenike J (2004) Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: molecular evidence from the host and parasite genomes. Genetics 168:1443–1445
Dyson EA, Hurst GDD (2004) Persistence of an extreme sex-ratio bias in a natural population. Proc Nat Acad Sci USA 101:6520–6523
Elnagdy S, Majerus MEN, Lawson Handley LJ (2011) The value of an egg: resource reallocation in ladybirds (Coleoptera: Coccinellidae) infected with male-killing bacteria. J Evol Biol 24:2164–2172
Engelstädter J, Telschow A, Hammerstein P (2004) Infection dynamics of different Wolbachia-types within one host population. J Theor Biol 231:345–355
Friberg U, Miller PM, Stewart AD, Rice WR (2011) Mechanisms promoting the long-term persistence of a Wolbachia infection in a laboratory-adapted population of Drosophila melanogaster. PLoS One 6:e16448
Fry AJ, Palmer MR, Rand DM (2004) Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 93:379–389
Goto S, Anbutsu H, Fukatsu T (2006) Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol 72:4805–4810
Haselkorn TS (2010) The Spiroplasma heritable bacterial endosymbiont of Drosophila. Fly 4:80–87
Haselkorn TS, Markow TA, Moran NA (2009) Multiple introductions of the Spiroplasma bacterial endosymbiont into Drosophila. Mol Ecol 18:1294–1305
Hedges LM, Brownlie JC, O'Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322:702–702
Hoffmann AA, Clancy DJ, Merton E (1994) Cytoplasmic incompatibility in Australian populations of Drosophila melanogaster. Genetics 136:993–999
Hoffmann AA, Hercus M, Dagher H (1998) Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148:221–232
Hurst GD, Jiggins FM (2000) Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg Infect Dis 6:329–336
Hurst GD, Johnson AP, vd Schulenburg JH, Fuyama Y (2000) Male-killing Wolbachia in Drosophila: a temperature-sensitive trait with a threshold bacterial density. Genetics 156:699–709
Hurst LD (1991) The incidences and evolution of cytoplasmic male killers. Proc R Soc Lond B Biol Sci 244:91–99
Hurst GDD, Majerus MEN (1993) Why do maternally inherited microorganisms kill males? Heredity 71:81–95
Ikeda H (1970) The cytoplasmically-inherited “sex-ratio” condition in natural and experimental populations of Drosophila bifasciata. Genetics 65:311–333
Ilinsky YY, Zakharov IK (2007) The endosymbiont Wolbachia in Eurasian populations of Drosophila melanogaster. Russ J Genet 43:748–756
Jaenike J, Polak M, Fiskin A, Helou M, Minhas M (2007) Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol Lett 3:23–25
Jaenike J, Stahlhut JK, Boelio LM, Unckless RL (2010) Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism? Mol Ecol 19:414–425
Kageyama D, Anbutsu H, Watada M, Hosokawa T, Shimada M, Fukatsu T (2006) Prevalence of a non-male-killing Spiroplasma in natural populations of Drosophila hydei. Appl Environ Microbiol 72:6667–6673
Kageyama D, Anbutsu H, Shimada M, Fukatsu T (2009) Effects of host genotype against the expression of spiroplasma-induced male killing in Drosophila melanogaster. Heredity 102:475–482
Martins A, Ventura IM, Klaczko LB (2010) Spiroplasma infection in Drosophila melanogaster: what is the advantage of killing males? J Inv Pathol 105:145–150
Malogolowkin C (1958) Maternally inherited “sex-ratio” conditions in Drosophila willistoni and Drosophila paulistorum. Genetics 43:274–286
Montenegro H, Klaczko LB (2004) Low temperature cure of a male killing agent in Drosophila melanogaster. J Inv Pathol 86:50–51
Montenegro H, Souza WN, da Silva LD, Klaczko LB (2000) Male-killing selfish cytoplasmic element causes sex-ratio distortion in Drosophila melanogaster. Heredity 85:465–470
Montenegro H, Solferini VN, Klaczko LB, Hurst GDD (2005) Male-killing Spiroplasma naturally infecting Drosophila melanogaster. Insect Mol Biol 14:281–287
Montenegro H, Hatadani LM, Medeiros HF, Klaczko LB (2006) Male killing in three species of the tripunctata radiation of Drosophila (Diptera: Drosophilidae). J Zool Syst 44:130–135
Montenegro H, Petherwick AS, Hurst GDD, Klaczko LB (2006) Fitness effects of Wolbachia and Spiroplasma in Drosophila melanogaster. Genetica 127:207–215
Osaka R, Nomura M, Watada M, Kageyama D (2008) Negative effects of low temperatures on the vertical transmission and infection density of a Spiroplasma endosymbiont in Drosophila hydei. Curr Microbiol 57:335–339
Pool JE, Wong A, Aquadro CF (2006) Finding of male-killing Spiroplasma infecting Drosophila melanogaster in Africa implies transatlantic migration of this endosymbiont. Heredity 97:27–32
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rubin G (1988) Drosophila melanogaster as an experimental organism. Science 240:1453–1459
Sachs JL, Mueller UG, Wilcox TP, Bull JJ (2004) The evolution of cooperation. Q Rev Biol 79:135–160
Thompson JD, Higgins DG, Gibson TJ (1994) ClustalW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Toju H, Fukatsu T (2011) Diversity and infection prevalence of endosymbionts in natural populations of the chestnut weevil: relevance of local climate and host plants. Mol Ecol 20:853–868
Vautrin E, Charles S, Genieys S, Vavre F (2007) Evolution and invasion dynamics of multiple infections with Wolbachia investigated using matrix based models. J Theor Biol 245:197–209
Vautrin E, Genieys S, Charles S, Vavre F (2008) Do vertically transmitted symbionts co-existing in a single host compete or cooperate? A modelling approach. J Evo Biol 21:145–161
Watts T, Haselkorn TS, Moran NA, Markow TA (2009) Variable incidence of Spiroplasma infections in natural populations of Drosophila species. PLoS One 4:e5703
White JA, Kelly SE, Cockburn PSJ, Hunter MS (2010) Endosymbiont costs and benefits in a parasitoid infected with both Wolbachia and Cardinium. Heredity 106:585–591
Williamson DL, Poulson DF (1979) Sex ratio organisms (spiroplasmas) of Drosophila. In: Whitcomb RF, Tully JG (eds) The mycoplasmas, vol 3. Academic Press, New York, pp 175–208
Williamson DL, Hackett KJ, Wagner AG, Cohen AJ (1989) Pathogenicity of cultivated Drosophila willistoni spiroplasmas. Curr Microbiol l9:53–56
Zhou W, Rousset F, O'Neil S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci 265:509–515
Acknowledgments
We thank Ana Teresa Brito, Lucilene Silva, Cynthia Brito, and Helvécio Santana for fly collections in Salvador and Paulo de Tarso Araripe, in Rio de Janeiro; Tamara Haselkorn for helpful tips; Julia Klaczko, Salete Couto, and Claudete Couto for technical help; and three anonymous referees for suggestions. Márcio José da Silva helped with the sequencing of DNA fragments. Financial support is received from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Fundação de Amparo à Pesquisa do Estado de São Paulo, and Fundo de Apoio ao Ensino, à Pesquisa e à Extensão–Unicamp.
Conflict of Interest
The authors declare no conflict of interest.
Data Archiving
Sequence data will be submitted to GenBank after the article acceptance, in accordance with the Instructions for Authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Iuri M. Ventura and Ayana B. Martins contributed equally to this work.
Electronic supplementary material
Supplementary information is available at Microbial Ecology's website.
ESM 1
(DOC 71 kb)
Rights and permissions
About this article
Cite this article
Ventura, I.M., Martins, A.B., Lyra, M.L. et al. Spiroplasma in Drosophila melanogaster Populations: Prevalence, Male-Killing, Molecular Identification, and No Association with Wolbachia . Microb Ecol 64, 794–801 (2012). https://doi.org/10.1007/s00248-012-0054-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-012-0054-6