Abstract
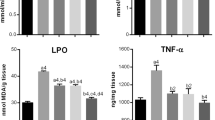
Extracorporeal shock-wave lithotripsy (ESWL)-induced renal damage can occur as a result of multiple mechanisms, including small vessel injury and free radical formation. Our previous studies have demonstrated that Astragalus membranaceus (AM), a traditional Chinese herb, could significantly alleviate shock wave-induced renal oxidative injury, and its renoprotective effects were superior to those of varapamil, a calcium antagonist, which were considered to be a powerful agent in treating renal damage during ESWL. However, the effective antioxidant ingredient of this herb in the setting of lithotripsy remains unclear. Astragalosides, the major components of AM, was demonstrated to have superior antioxidation properties both in vitro and in vivo. Therefore, in this study we further investigate the potential effects of astragalosides on the shock wave-induced oxidative stress in rabbit kidney. Thirty male rabbits were randomly assigned to two groups, each consisting of 15 rabbits: (1) control group, (2) astragaloside-treated group. Each group of animals underwent 1,500 shock waves to the right kidney. Peripheral blood, urine and kidney tissue samples were collected pre- and post-ESWL. The level of urinary N-acetyl-β-glucosaminidase (NAG), serum creatinine, serum or homogenates malondialdehyde (MDA) and superoxide dismutase (SOD), respectively, were detected. Histological alterations were also examined through light microcopy and transmission electron microscopy. In the control group, shock wave significantly increased the level of MDA and decreased SOD activity in both blood and renal homogenates (P < 0.05, respectively). The comparison between the control and astragalosides group demonstrated that astragalosides could significantly decrease the level of MDA (P < 0.05) and inhibit the decline of SOD activity (P < 0.05). After exposure to shock waves, the activity of urinary NAG increased significantly in the control group (P < 0.05). However, the concentration of serum creatinine did not change significantly. The comparison between the control and astragalosides group demonstrated that astragalosides significantly reduced the shock wave-induced leakage of NAG into the urine (P < 0.05). Histological examination also showed that renal morphological impairments were much milder in astragaloside-treated rabbits than those of the control group. Our results indicated that astragaloside treatment provided significant protection against shock wave-induced renal oxidative injury.


Similar content being viewed by others
References
Evan AP, Wills LR, McAteer JA, Bailey MR, Connors BA, Shao Y, Lingeman JE, Williams JC Jr, Finberg NS, Crum LA (2002) Kidney damage and renal functional changes are minimized by waveform control that suppresses cavitation in shock wave lithotripsy. J Urol 168:1556–1562
Jaeger P, Redha F, Marquardt K, Uhlschmid G, Hauri D (1995) Morphological and functional changes in canine kidneys following extracorporeal shock wave treatment. Urol Int 54:48–58
Karlsen SJ, Smevik B, Hovig T (1991) Acute morphological changes in canine kidneys after exposure to extracorporeal shock waves: a light and electron microscopic study. Urol Res 19:105–115
Villanyi KK, Szekely JG, Farkas LM, Javor E, Pusztai C (2001) Short-term changes in renal function after extracorporeal shock wave lithotripsy in children. J Urol 166:222–224
Lipski B, Miller J, Rigaud G, Stack G, Marsh C (1997) Acute renal failure from a subcapsular hematoma in a solitary kidney: an usual complication of extracorporeal shock wave lithotripsy. J Urol 157:224–225
Williams JC Jr, Stonehill MA, Colmenares K, Evan AP, Andreoli SP, Cleveland RO, Bailey MR, Crum LA, McAteer JA (1999) Effect of macroscopic air bubbles on cell lysis by shock wave lithotripsy in vitro. Ultrasound Med Biol 25:473–479
Delvecchio F, Auge BK, Munver R, Brown SA, Brizuela R, Zhong P, Preminger GM (2003) shock wave lithotripsy causes ipsilateral renal injury remote from the focal point: the role of regional vasoconstriction. J Urol 169:1526–1529
Munver R, Delvecchio F, Kuo RL, Brown SA, Zhong P, Preminger GM (2002) In vivo assessment of free radical activity during shock wave lithotripsy using a microdialysis system: the renoprotective action of allopurinol. J Urol 167:327–334
Biri H, Ozturk HS, Buykkocak S, Kacmaz M, Cimen MY, Unal D, Birey M, Bozkirli I, Durak I (1998) Antioxidant defense potential of rabbit renal tissue after ESWL: protective effects of antioxidant vitamins. Nephron 79:181–185
Serel TA, Ozguner F, Soyupek S (2004) Prevention of shock wave induced renal oxidative stress by melatonin: an experimental study. Urol Res 32:69–71
Tada S, Yase Y, Shirataki Y (2000) Inhibitory effects of astragali radix, crude drug in Oriental medicines on lipid peroxidation and protein oxidative modification of mouse brain homogenates by copper. Phytother Res 14:294–296
Hei ZQ, Huang HQ, Zhang JJ, Cheng BX, Li XY (2005) Protective effects of astragalus membranaceus on intestinal mucosa reperfusion injury after hemorrhagic shock in rats. World J Gastroenterol 11:4986–4991
Jia RZ, Jiang L, Qiao LX (2005) Study on effect of radix astragali on injury of cerebral cortex in neonatal rats after hypoxia/ischemia brain damage. Zhongguo Zhong Xi Yi Jie He Za Zhi 25:54–57
Sheng BW, Chen XF, Zhao J, He DL, Nan XY (2005) Astragalus membranaceus reduces free radical-mediated renal tubules injury in rabbits receiving High energy shock waves. Chin Med J 118:43–49
Wang D, Shen W, Tian Y, Sun Z, Jiang C, Yuan S (1996) Protective effect of active components extracted from radix Astragali on human erythrocyte membrane damage caused by reactive oxygen species. Zhongguo Zhong Yao Za Zhi 21:746–748
Lei H, Wang B, Li WP, Yang Y, Zhou AW, Chen MZ (2003) Anti-aging effect of Astragalosides and its mechanism of action. Acta Pharmacol Sin 24:230–234
Lu S, Zhang J, Yang D (1999) Effects of Astragaloside in treating myocardial injury and myocardial Sarco/Endoplasmic Ca2+_ATPase of viral myocarditis mice. Zhongguo Zhong Yi Xi Jie He Za Zhi 19:672–674
Meng D, Chen XJ, Bian YY, Li P, Yang D, Zhang JN (2005) Effect of Astragaloside on intracellular calcium overload in cultured cardiac myocytes of neonatal rats. Am J Chin Med 33:11–20
Zhou JY, Fan Y, Kong JL, Wu DZ, Hu ZB (2000) Effects of components isolated from Astragalus membranaceus Bunge on cardiac function injured by myocardial ischemia reperfusion in rats. Zhongguo Zhong Yao Za Zhi 25:300–302
Sun XZ, Zhang ZW (2004) Domestic shock wave lithotripters and their characteristics. Chin Med Equip J 8:28–29
Koo DD, Welsh KI, West NE, Channon KM, Penington AJ, Roake JA, Morris PJ, Fuggle SV (2001) Endothelial cell protection against ischemia reperfusion injury by lecithinized superoxide dismutase. Kidney Int 60:786–796
Noto A, Ogawa Y, Mori S, Yoshioka M, Kitakaze T, Hori T, Nakamura M, Miyake T (1983) Simple, rapid spectrophotometry of urinary N-acetyl-beta-D-glucosaminidase, with use of a new chromogenic substrate. Clin Chem 29:1713–1716
Benigni A, Corna D, Maffi R, Benedetti G, Zoja C, Remuzzi G (1998) Renoprotective effect of contemporary blocking of angiotensin II and endothelin-1 in rats with membranous nephropathy. Kidney Int 54:353–359
Tamada S, Nakatani T, Asai T, Tashiro K, Komiya T, Sumi T, Okamura M, Kim S, Iwao H, Kishimoto T, Yamanaka S, Miura K (2003) Inhibition of nuclear factor-κB activation by pyrrolidine dithiocarbamate prevents chronic FK506 nephropathy. Kidney Int 63:306–314
Knap PM, Kulb PB, Lingeman JE, Newman DM, Mertz JH, Mosbaugh PG, Steele RE (1988) Extra corporeal shock wave lithotripsy induced perirenal hematomas. J Urol 139:700–703
Rubin JI, Arger PH, Pollack HM, Banner MP, Coleman BG, Mintz MC, Van Arsdalen KN (1987) Kidney changes after shock wave lithotripsy: CT evaluation. Radiology 162:21–24
Strohmaier WL, Billes IC, Abelius A, Lahme S, Bichler KH (2002) Selenium reduces high energy shock wave induced renal injury in rats. Urol Res 30:31–34
Ogiste JS, Nejat RJ, Rashid HH, Greene T, Gupta M (2003) The role of mannitol in alleviating renal injury during extracorporeal shock wave lithotripsy. J Urol 169:875–877
Suhr D, Brummer F, Hulcer DF (1991) Cavitation-generated free radicals during shock wave exposure: investigations with cell-free solutions and suspended cells. Ultrasound Med Biol 17: 761–768
Cohen TD, Durrani AF, Brown SA, Ferraro R, Preminger GM (1998) Lipid peroxidation induced by shockwave lithotripsy. J Endourol 12:229–232
Sarica K, Kosar A, Yaman O, Beduk Y, Durak I, Gogus O, Kavukcu M (1996) Evaluation of ischemia after ESWL: detection of oxygen free radical scavenger enzymes in renal parenchyma subjected to high energy shock waves. Urol Int 57:221–223
Defraigne JO, Detry O, Pincemail J, Franssen C, Meurisse M, Lamy M, Limet R (1994) Direct evidence of free radical production after ischemia and reperfusion and protective effect of desferrioxamine: ESR and vitamin E studies. Eur J Vasc Surg 8:537–543
Boaz M, Matas Z, Biro A, Katzir Z, Green M, Fainaru M, Smetana S (1999) Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int 56: 1078–1083
Vaziri ND, Lin CY, Farmand F, Sindhu RK (2003) Superoxide dismutase, catalase, glutathione peroxidase and NADPH oxidase in lead-induced hypertension. Kidney Int 63:186–194
Ozguner F, Armagan A, Koyu A, Caliskan S, Koylu H (2005) A novel antioxidant agent caffeic acid phenethyl ester prevents shock wave-induced renal tubular oxidative stress. Urol Res 33:239–243
Yaman O, Sarica K, Ozer G, Soygur T, Kutsal O, Yaman LS, Gous O (1996) Protective effect of varapamil on renal tissue during shock wave application in rabbit kidney. J Endourol 10:329–333
Walter LS, Inge CB, Annette A, Sven L, Karl-Horst B (2002) Selenium reduces high energy shock wave-induced renal injury in rats. Urol Res 30:31–34
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, X., He, D., Zhang, L. et al. A novel antioxidant agent, astragalosides, prevents shock wave-induced renal oxidative injury in rabbits. Urol Res 34, 277–282 (2006). https://doi.org/10.1007/s00240-006-0057-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-006-0057-1