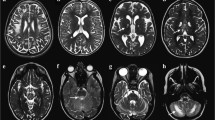
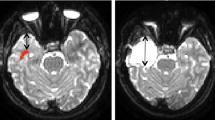
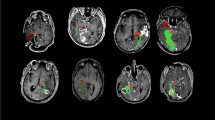
Abstract
Purpose
This study assessed whether optic radiations (OR) microstructure after temporal lobe epilepsy (TLE) surgery correlated with visual field defects (VFD).
Methods
Patients were subjected to diffusion tensor imaging (DTI) tractography of the OR and Humphrey perimetry after TLE surgery. We used Spearman’s test to verify correlations between tractographic parameters and perimetry mean deviation. Tractographic variables were compared between patients with VFD or intact perimetry. Multiple logistic regression was applied between DTI and perimetry values. DTI sensitivity and specificity were assessed with a receiver operating characteristic (ROC) curve to evaluate VFD.
Results
Thirty-nine patients had reliable perimetry and OR tractography. There was a significant correlation between (1) fractional anisotropy (FA) and both total (rho = 0.569, p = 0.0002) and quadrant (rho = 0.453, p = 0.0037) mean deviation and (2) radial diffusivity and total mean deviation (rho = − 0.350, p = 0.0286). There was no other significant correlation. Patients with VFD showed a significantly lower FA compared with patients with normal perimetry (p = 0.0055), and a 0.01 reduction in FA was associated with a 44% increase in presenting VFD after surgery (confidence interval, CI = 1.10–1.88; p = 0.0082). Using a FA of 0.457, DTI tractography showed a specificity of 95.2% and a sensitivity of 50% to detect VFD after surgery (area under the curve = 0.7619, CI = 0.6020–0.9218).
Conclusion
The postoperative OR microstructure correlated with visual loss after epilepsy surgery. DTI postoperative OR tractography may be helpful in evaluating VFD.






Similar content being viewed by others
References
Wiebe S, Blume WT, Girvin JP, Eliasziw M (2001) A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med 345:311–318. https://doi.org/10.1056/NEJM200108023450501
Asadi-Pooya AA, Stewart GR, Abrams DJ, Sharan A (2017) Prevalence and incidence of drug-resistant mesial temporal lobe epilepsy in the United States. World Neurosurg 99:662–666. https://doi.org/10.1016/j.wneu.2016.12.074
Cendes F (2005) Mesial temporal lobe epilepsy syndrome: an updated overview. J Epilepsy Clin Neurophysiol 11:141–144. https://doi.org/10.1590/S1676-26492005000300006
Tecoma ES, Laxer KD, Barbaro NM, Plant GT (1993) Frequency and characteristics of visual field deficits after surgery for mesial temporal sclerosis. Neurology 43:1235–1238
Egan RA, Shults WT, So N, Burchiel K, Kellogg JX, Salinsky M (2000) Visual field deficits in conventional anterior temporal lobectomy versus amygdalohippocampectomy. Neurology 55:1818–1822
Yogarajah M, Focke NK, Bonelli S, Cercignani M, Acheson J, Parker GJM, Alexander DC, McEvoy AW, Symms MR, Koepp MJ, Duncan JS (2009) Defining Meyer’s loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain 132:1656–1668. https://doi.org/10.1093/brain/awp114
Dreessen de Gervai P, Sboto-Frankenstein UN, Bolster RB, Thind S, Gruwel MLH, Smith SD, Tomanek B (2014) Tractography of Meyer’s loop asymmetries. Epilepsy Res 108:872–882. https://doi.org/10.1016/j.eplepsyres.2014.03.006
Yeni SN, Tanriover N, Uyanik Ö, Ulu MO, Özkara Ç, Karaağaç N, Ozyurt E, Uzan M (2008) Visual field defects in selective amygdalohippocampectomy for hippocampal sclerosis: the fate of Meyer’s loop during the Transsylvian approach to the temporal horn. Neurosurgery 63:507–515. https://doi.org/10.1227/01.NEU.0000324895.19708.68
Winston GP, Mancini L, Stretton J, Ashmore J, Symms MR, Duncan JS, Yousry TA (2011) Diffusion tensor imaging tractography of the optic radiation for epilepsy surgical planning: a comparison of two methods. Epilepsy Res 97:124–132. https://doi.org/10.1016/j.eplepsyres.2011.07.019
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265. https://doi.org/10.3978/j.issn.2223-4292.2012.12.05
van Lanen RHGJ, Hoeberigs MC, Bauer NJC, Haeren RHL, Hoogland G, Colon A, Piersma C, Dings JTA, Schijns OEMG (2018) Visual field deficits after epilepsy surgery: a new quantitative scoring method. Acta Neurochir 160:1325–1336. https://doi.org/10.1007/s00701-018-3525-9
Winston GP, Mancini L, Stretton J, Ashmore J, Symms MR, Duncan JS, Yousry TA (2011) Diffusion tensor imaging tractography of the optic radiation for epilepsy surgical planning: a comparison of two methods. Epilepsy Res 97:124–132. https://doi.org/10.1016/j.eplepsyres.2011.07.019
Winston GP (2013) Epilepsy surgery, vision, and driving: what has surgery taught us and could modern imaging reduce the risk of visual deficits? Epilepsia 54:1877–1888. https://doi.org/10.1111/epi.12372
Winston GP, Daga P, Stretton J, Modat M, Symms MR, McEvoy AW, Ourselin S, Duncan JS (2012) Optic radiation tractography and vision in anterior temporal lobe resection. Ann Neurol 71:334–341. https://doi.org/10.1002/ana.22619
Lilja Y, Nilsson DT (2015) Strengths and limitations of tractography methods to identify the optic radiation for epilepsy surgery. Quant Imaging Med Surg 5:288–299. https://doi.org/10.3978/j.issn.2223-4292.2015.01.08
Delev D, Wabbels B, Schramm J, Nelles M, Elger CE, von Lehe M, Clusmann H, Grote A (2016) Vision after trans-sylvian or temporobasal selective amygdalohippocampectomy: a prospective randomised trial. Acta Neurochir 158:1757–1765. https://doi.org/10.1007/s00701-016-2860-y
Yaşargil MG, Wieser HG, Valavanis A, von Ammon K, Roth P (1993) Surgery and results of selective amygdala-hippocampectomy in one hundred patients with nonlesional limbic epilepsy. Neurosurg Clin N Am 4:243–261
Yaşargil MG, Teddy PJ, Roth P (1985) Selective amygdalo-hippocampectomy. Operative anatomy and surgical technique. Adv Tech Stand Neurosurg 12:93–123
Ghizoni E, Almeida J, Joaquim A, Yasuda C, de Campos B, Tedeschi H, Cendes F (2015) Modified anterior temporal lobectomy: anatomical landmarks and operative technique. J Neurol Surg Part A Cent Eur Neurosurg 76:407–414. https://doi.org/10.1055/s-0035-1549303
Ghizoni E, Matias RN, Lieber S, de Campos BM, Yasuda CL, de Souza JPSAS, Pereira PC, Amato Filho ACS, Joaquim AF, Lopes TM, Tedeschi H, Cendes F (2017) Clinical and imaging evaluation of transuncus selective amygdalohippocampectomy. World Neurosurg 100:665–674. https://doi.org/10.1016/j.wneu.2016.11.056
Kwan P, Schachter SC, Brodie MJ (2011) Drug-resistant epilepsy. N Engl J Med 365:919–926. https://doi.org/10.1056/NEJMra1004418
Walsh TJ (2011) Visual fields: examination and interpretation. Oxford University Press, New York
Hervás Navidad R, Altuzarra Corral A, Lucena Martín JA et al (2002) Defectos del campo visual en la cirugía resectiva de la epilepsia del lóbulo temporal. Rev Neurol 34:1025. https://doi.org/10.33588/rn.3411.2001505
Pathak-Ray V, Ray A, Walters R, Hatfield R (2002) Detection of visual field defects in patients after anterior temporal lobectomy for mesial temporal sclerosis-establishing eligibility to drive. Eye (Lond) 16:744–748. https://doi.org/10.1038/sj.eye.6700152
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265. https://doi.org/10.3978/j.issn.2223-4292.2012.12.05
Winston GP, Stretton J, Sidhu MK, Symms MR, Duncan JS (2014) Progressive white matter changes following anterior temporal lobe resection for epilepsy. NeuroImage Clin 4:190–200. https://doi.org/10.1016/j.nicl.2013.12.004
Hughes TS, Abou-Khalil B, Lavin PJ et al (1999) Visual field defects after temporal lobe resection: a prospective quantitative analysis. Neurology 53:167–172
Párraga RG, Ribas GC, Welling LC et al (2012) Microsurgical anatomy of the optic radiation and related fibers in 3-dimensional images. Oper Neurosurg 71:ons160–ons172. https://doi.org/10.1227/NEU.0b013e3182556fde
Hofer S, Karaus A, Frahm J (2010) Reconstruction and dissection of the entire human visual pathway using diffusion tensor MRI. Front Neuroanat 4:15. https://doi.org/10.3389/fnana.2010.00015
Winston GP, Daga P, White MJ, Micallef C, Miserocchi A, Mancini L, Modat M, Stretton J, Sidhu MK, Symms MR, Lythgoe DJ, Thornton J, Yousry TA, Ourselin S, Duncan JS, McEvoy AW (2014) Preventing visual field deficits from neurosurgery. Neurology 83:604–611. https://doi.org/10.1212/WNL.0000000000000685
Winston GP (2013) Epilepsy surgery, vision, and driving: what has surgery taught us and could modern imaging reduce the risk of visual deficits? Epilepsia 54:1877–1888. https://doi.org/10.1111/epi.12372
Heijl A (1989) The effect of perimetric experience in normal subjects. Arch Ophthalmol 107:81. https://doi.org/10.1001/archopht.1989.01070010083032
Bengtsson B, Heijl A (1999) Inter-subject variability and normal limits of the SITA standard, SITA fast, and the Humphrey full threshold computerized perimetry strategies, SITA STATPAC. Acta Ophthalmol Scand 77:125–129
Wild JM, Pacey IE, O’Neill EC, Cunliffe IA (1999) The SITA perimetric threshold algorithms in glaucoma. Invest Ophthalmol Vis Sci 40:1998–2009
Budenz DL, Rhee P, Feuer WJ et al (2002) Comparison of glaucomatous visual field defects using standard full threshold and Swedish interactive threshold algorithms. Arch Ophthalmol (Chicago, Ill 1960) 120:1136–1141
Budenz DL, Rhee P, Feuer WJ, McSoley J, Johnson CA, Anderson DR (2002) Sensitivity and specificity of the Swedish interactive threshold algorithm for glaucomatous visual field defects. Ophthalmology 109:1052–1058
Sekhar GC, Naduvilath TJ, Lakkai M et al (2000) Sensitivity of Swedish interactive threshold algorithm compared with standard full threshold algorithm in Humphrey visual field testing. Ophthalmology 107:1303–1308
Wild JM, Pacey IE, Hancock SA, Cunliffe IA (1999) Between-algorithm, between-individual differences in normal perimetric sensitivity: full threshold, FASTPAC, and SITA. Swedish Interactive Threshold algorithm. Invest Ophthalmol Vis Sci 40:1152–1161
Artes PH, Iwase A, Ohno Y, Kitazawa Y, Chauhan BC (2002) Properties of perimetric threshold estimates from full threshold, SITA standard, and SITA fast strategies. Invest Ophthalmol Vis Sci 43:2654–2659
Bengtsson B, Heijl A (1998) Evaluation of a new perimetric threshold strategy, SITA, in patients with manifest and suspect glaucoma. Acta Ophthalmol Scand 76:268–272
Shirato S, Inoue R, Fukushima K, Suzuki Y (1999) Clinical evaluation of SITA: a new family of perimetric testing strategies. Graefes Arch Clin Exp Ophthalmol 237:29–34
Aydin A, Kocak I, Aykan U, Can G, Sabahyildizi M, Ersanli D (2015) The influence of the learning effect on automated perimetry in a Turkish population. J Fr Ophtalmol 38:628–632. https://doi.org/10.1016/j.jfo.2015.01.013
Castro DPE, Kawase J, Melo LAS (2008) Learning effect of standard automated perimetry in healthy individuals. Arq Bras Oftalmol 71:523–528
Fraser JA, Newman NJ, Biousse V (2011) Disorders of the optic tract, radiation, and occipital lobe. Handb Clin Neurol 102:205–221. https://doi.org/10.1016/B978-0-444-52903-9.00014-5
Funding
This work was funded by the São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM. 1
Scatterplots that show the distribution of DTI and perimetry variables according to the surgical approach. Red dots and blue triangles represent patients who underwent modified anterior temporal lobectomy (mATL) and the transsylvian approach, respectively (PDF 474 kb)
Rights and permissions
About this article
Cite this article
de Souza, J.P.S.A.S., Ayub, G., Pereira, P.C. et al. Fractional anisotropy of the optic radiations correlates with the visual field after epilepsy surgery. Neuroradiology 61, 1425–1436 (2019). https://doi.org/10.1007/s00234-019-02281-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-019-02281-2