Abstract
Introduction
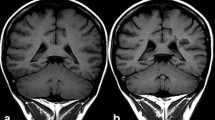
Gadolinium-based contrast agents (GBCAs) have been used clinically since 1988 for contrast-enhanced magnetic resonance imaging (CE-MRI). Generally, GBCAs are considered to have an excellent safety profile. However, GBCA administration has been associated with increased occurrence of nephrogenic systemic fibrosis (NSF) in patients with severely compromised renal function, and several studies have shown evidence of gadolinium deposition in specific brain structures, the globus pallidus and dentate nucleus, in patients with normal renal function.
Methods
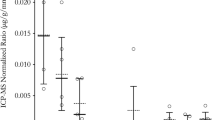
Gadolinium deposition in the brain following repeated CE-MRI scans has been demonstrated in patients using T1-weighted unenhanced MRI and inductively coupled plasma mass spectroscopy. Additionally, rodent studies with controlled GBCA administration also resulted in neural gadolinium deposits.
Results
Repeated GBCA use is associated with gadolinium deposition in the brain. This is especially true with the use of less-stable, linear GBCAs. In spite of increasing evidence of gadolinium deposits in the brains of patients after multiple GBCA administrations, the clinical significance of these deposits continues to be unclear.
Conclusion
Here, we discuss the current state of scientific evidence surrounding gadolinium deposition in the brain following GBCA use, and the potential clinical significance of gadolinium deposition. There is considerable need for further research, both to understand the mechanism by which gadolinium deposition in the brain occurs and how it affects the patients in which it occurs.
Similar content being viewed by others
References
Kanal E, Maravilla K, Rowley HA (2014) Gadolinium contrast agents for CNS imaging: current concepts and clinical evidence. AJNR Am J Neuroradiol 35:2215–2226
Zhou Z, Lu ZR (2013) Gadolinium-based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5:1–18
Giesel F, Mehndiratta A, Essig M (2010) High-relaxivity contrast enhanced magnetic resonance neuroimaging: a review. Eur Radiol 20:2461–2474
Grobner T (2006) Gadolinium—a specific trigger of development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108
Marckmann P, Skov L, Rosen K et al (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17:2359–2362
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Errante Y, Cirimele V, Mallio CA et al (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:800–809
Quattrocchi CC, Malio CA, Errante Y et al (2015) Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Investig Radiol 50:470–472
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Jubisavljevic S (2015) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol.
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruvama T, Kitajima K, Furui S (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1):228–32
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, Semelka RC (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276(3):836–44
Weberling L, Kieslich P, Kickingereder P et al (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Investig Radiol 50(11):743–48
Roberts D and Holden K (2015) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev.
Radbruch A, Weberling L, Kieslich P et al (2015) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images. Investig Radiol 50(12):805–810
Cao Y, Huang DQ, Shih G, Prince MR (2016) Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopenetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206(2):414–9
Robert P, Lehericy S, Grand S, Violas X, Fratellier N, Idee JM, Ballet S, Corot C (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats. Investig Radiol 50(8):473–480
Robert P, Violas X, Grand S, et al. (2015) Linear gadolinium-based contrast agents are associated with brain gadolinium retention in healthy rats. Invest Radiol.
Jost G, Lenhard DC, Sieber MA et al (2016) Signal increase on unenhanced T1-weighted images in the Rat brain after repeated, extended doses of gadolinium-based contrast agents: comparison of linear and macrocyclic agents. Investig Radiol 51(2):83–9
United States Food and Drug Administration (2015) http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm456012.htm
Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M (2012) MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging 36(5):1060–1071
Bellin MF, Van der Molen AJ (2008) Extracellular gadolinium-based contrast media: an overview. Eur Radiol 66:160–167
Caravan P, Ellison JJ, Mcmurry TJ, Lauferr JB (1999) Gadolinium(III) chelates as MRI contrast agents: structures, dynamics and applications. Chem Rev 99:2293–2352
Bellin MF, Vasile M, Morel-Prtecetti S (2003) Currently used non-specific extracellular MR contrast media. Eur Radiol 13:2688–2698
Aime S, Caravan P (2009) Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging 30:1259–1267
Pietsch H, Lengsfeld P, Jost G et al (2009) Long term retention of gadolinium in the skin of following the administration of gadolinium based contrast agents. Eur Radiol 19:1417–1424
Pirovano G, Munley J, Schultz C et al (2012) Nephrogenic systemic fibrosis: a review of published cases and results from three prospective observational studies. Insights Imaging 3(suppl 1):S293
Martin DR, Krishnamoorthy SK, Kalb B et al (2010) Decreased incidence of NSF in patients on dialysis after changing gadolinium contrast-enhanced MRI protocols. J Magn Reson Imaging 31:440–446
Bennett CL, Qureshi ZP, Sartor AO et al (2012) Gadolinium-induced nephrogenic systemic fibrosis: the rise and fall of an iatrogenic disease. Clin Kidney J 5:82–88
Abujudeh HH, Kosaraju VK, Kaewlai R (2010) Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 21,659 injections. AJR Am J Roentgentol 194:430–34
American College of Radiology. Manual on Contrast Media. Version 10.1. 2015. http://www.acr.org
Bruder O, Schneider S, Nothnagel D et al (2011) Acute adverse reactions to gadolinium-based contrast agents in CMR: multicenter experience with 17,767 patients from the EuroCMR Registry. JACC Cardiovasc Imaging 4:1171–76
Cochran ST, Bomyea K, Sayre JW (2001) Trends in adverse events after IV administration of contrast media. AJR Am J Roentgenol 176:1385–88
Forsting M, Palkowitsch P (2010) Prevalence of acute adverse reactions to gadobutrol: a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur J Radiol 74:e186–92
Murphy KP, Szopinski KT, Cohal RH et al (1999) Occurrence of adverse reactions to gadolinium-based contrast material and management of patients at increased risk: a survey of the American Society of Neuroradiology Fellowship Directors. Acad Radiol 6:656–64
Li A, Wong CS, Wong MK et al (2006) Acute adverse reactions to magnetic resonance contrast media: gadolinium chelates. Br J Radiol 79:368–71
Dillman JR, Ellis JH, Cohan RH et al (2007) Frequency and severity of acute allergic-like reactions to gadolinium-containing I.V. contrast media in children and adults. AJR Am J Roentgenol 189:1533–38
Hunt CH, Hartman RP, Hesley GK (2009) Frequency and severity of adverse effects of iodinated and gadolinium contrast materials: retrospective review of 456,930 doses. AJR Am J Roentgenol 193:1124–27
Prince MR, Zhang H, Zou Z et al (2011) Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 196:W138–43
Jung JW, Kang HR, Kim MH et al (2012) Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 264:414–22
Vervloet D, Durham S (1998) Adverse reactions to drugs. BMJ 316:1511–14
Hasdenteufel F, Luyasu S, Renaudin JM et al (2008) Anaphylactic shock after first exposure to gadoterate meglumine: two case reports documented by positive allergy assessment. J Allergy Clin Immunol 121:527–28
Schiavino D, Murzilli F, Del Ninno M et al (2003) Demonstration of an IgE-mediated immunological pathogenesis of a severe reaction to gadopentetate dimeglumine. J Investig Allergol Clin Immunol 13:140–42
Fakhran S, Alhilali L, Kale H, Kanal E (2015) Assessment of rates of acute adverse reactions to gadobenate dimeglumine: review of more than 130,000 administrations in 7.5 years. AJR Am J Roentgenol 204(4):703–6
Beomonte Zobel B, Quattrocchi CC, Errante Y, Grasso RF (2015) Gadolinium-based contrast agents: did we miss something in the last 25 years? Radiol Med
Ramalho J, Semelka RC, Ramalho M, et al. (2015) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol
Thomsen HS (2016) T1 hyperintensity in the brain after multiple intravenous injections of gadolinium-based contrast agents. Acta Radiol
Runge VM (2015) Macrocyclic versus linear gadolinium chelates. Investig Radiol 50(12):811
Agris J, Pietsch H, and Balzer T (2015) What evidence is there that gadobutrol causes increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W MRI in patients with RRMS? Eur Radiol
Stojanov D (2015) Reply to Letter to the Editor re: Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol
Bussi S, Fouillet X, Morisetti A (2007) Toxicological assessment of gadolinium release from contrast media. Exp Toxicol Pathol 58:323–330
Sieber MA, Pietsch H, Walter J et al (2008) Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in-vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 18:2164–2173
Frenzel T, Lengsfeld P, Schirmer H et al (2008) Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Investig Radiol 43:817–828
Idée JM, Port M, Dencausse A et al (2009) Involvement of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: an update. Radiol Clin North Am 47:855–869
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41(3):272–278
Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE (2009) Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 1(6):479–488
Ogi S, Fukumitsu N, Tsuchida D, Uchiyama M, Mori Y, Matsui K (2002) Imaging of bilateral striopallidodentate calcinosis. Clin Nucl Med 27(10):721–724
Tabanor K, Lee P, Kiptoo P, et al. (2016) Brain delivery of drug and MRI contrast agent: detection and quantitative determination of brain deposition of CPT-Glu using LC-MS/MS and Gd-DTPA using magnetic resonance imaging. Mol Pharm
Runge VM (2015) Commentary on T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats. Investig Radiol 50(8):481–82
Sanyal S, Marckmann P, Scherer S et al (2011) Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis—an autopsy-based review. Nephrol Dial Transplant 26:3616–3626
Roccatagliata L, Vuolo L, Bonzano L et al (2009) Multiple sclerosis: hyperintense dentate nucleus on unenhanced T1-weighted images is associated with the secondary progressive subtype. Radiology 251:503–510
LeVine SM (1997) Iron deposits in multiple sclerosis and Alzheimer’s disease brains. Brain Res 760:298–303
Chan DE, Pan HC, Ho DM et al (2007) Presence of activated microglia in a high-signal lesion on T1-weighted MR images: a biopsy sample re-examined. AJNR Am J Neuroradiol 28:602
Drayer B, Burger P, Hurwitz B et al (1987) Reduced signal intensity on MR images of thalamus and putamen in multiple sclerosis: increased iron content? AJR Am J Roentgenol 149:357–363
Brass SD, Chen NK, Mulkern NV, Bakshi R (2006) Magnetic resonance imaging of iron deposition in neurological disorders. Top Magn Reson Imaging 17:31–40
Drayer BP, Burger P, Hurwtz B et al (1987) Magnetic resonance imaging in multiple sclerosis: decreased signal in thalamus and putamen. Ann Neurol 22:546–550
Craelius W, Migdal MW, Luessenhop CP et al (1982) Iron deposits surrounding multiple sclerosis plaques. Arch Pathol Lab Med 106:397–399
Shin JC, Kim E, Sheong HK et al (2007) High signal intensity on magnetic resonance imaging as a predictor of neurobehavioral performance of workers exposed to manganese. Neurotoxicology 28:257–262
Fujioka M, Taoka T, Matsuo Y et al (2003) Magnetic resonance imaging shows delayed ischemic striatal neurodegeneration. Ann Neurol 54:732–747
Powell T, Sussman JG, Davies-Jones GA (1992) MR imaging in acute multiple sclerosis: ring-like appearance in plaque suggesting the presence of paramagnetic free radicals. AJNR Am J Neuroradiol 13:1544–1546
Terada H, Barkovich AJ, Edwards MS, Ciricillo SM (1996) Evolution of high-intensity basal ganglia lesions on T1-weighted MR in neurofibromatosis type 1. AJNR Am J Neuroradiol 17:755–760
Daszkiewicz OK, Hennel JW, Szczepkowski TW, Lubas B (1963) Proton magnetic relaxation and protein hydration. Nature 200:1006–1007
Henkelman RM, Watts JF, Kucharzyk W (1991) High signal intensity in MR images of calcified brain tissue. Radiology 179:199–206
Boyko OB, Burger PC, Shelburne JD, Ingram P (1992) Non-heme mechanisms for T1 shortening: pathologic, CT, and MR elucidation. AJNR Am J Neuroradiol 13:1439–1445
Warakaulle DR, Anslow P (2003) Differential diagnosis of intracranial lesions with high signal on T1 or low signal on T-2 weighted MRI. Clin Radiol 58:922–933
Suzuki S, Nishio S, Takata K et al (2000) Radiation-induced brain calcification: paradoxical high signal intensity in T1-weighted images. Acta Neurochir (Wien) 142:801–804
Weinmann HJ, Gries H, Speck U (1992) Fundamental physics and chemistry: types of contrast agents. In: Sartor K (ed) MR imaging of the skull and brain: a correlative text atlas. Springer-Verlag, New York, pp 26–28
Hegde A, Mohan S, Lath N, Lim CCT (2011) Differential diagnosis for bilateral abnormalities of basal ganglia and thalamus. Radiographics 31:5–30
Kim TJ, Kim TO, Kim WS et al (2006) MR imaging of the brain in Wilson disease of childhood: findings before and after treatment with clinical correlation. AJNR Am J Neuroradiol 2(6):1373–1378
Lai PH, Chen C, Liang HL, Pan HB (1999) Hyperintense basal ganglia on T1-weighted MR imaging. AJNR Am J Neuroradiol 172(4):1109–1115
Valdés Hernández Mdel C, Maconick LC, Tan EM, Wardlaw JM (2012) Identification of mineral deposits in the brain on radiological images: a systematic review. Eur Radiol 22(11):2371–2381
Brunberg JA, Kanal E, Hirsch W, Van Thiel DH (1991) Chronic acquired hepatic failure: MR imaging of the brain at 1.5 T. AJNR Am J Neuroradiol 12(5):909–914
Rovira A, Alonso J, Cordoba J (2008) MR imaging findings in hepatic encephalopathy. AJNR Am J Neuroradiol 29(9):1612–1621
Oikonomu A, Chatzistefanou A, Zezos P et al (2012) Basal ganglia hyperintensity on T1-weighted MRI in Rendu-Osler-Weber disease. J Magn Res Imaging 35(2):426–430
Mirowitz SA, Westicks TJ, Hirsch JD (1991) Hyperintense basal ganglia on T1-weighted MR images in patients receiving parenteral nutrition. Radiology 181(1):117–120
da Silva CJ, da Rocha AJ, Jeronymo S et al (2007) A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: manganism symptoms and T1 hyperintense changes in the basal ganglia. AJNR Am J Neuroradiol 28(8):1474–1479
Martin-Duverneuil N, Idbaih A, Hoang-Xuan K et al (2006) MRI features of neurodegenerative Langerhans cell histiocytosis. Eur Radiol 16(9):2074–2082
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that this manuscript does not contain clinical studies or patient data.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Stojanov, D., Aracki-Trenkic, A. & Benedeto-Stojanov, D. Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents—current status. Neuroradiology 58, 433–441 (2016). https://doi.org/10.1007/s00234-016-1658-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1658-1