Abstract
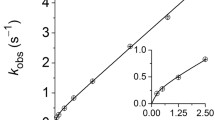
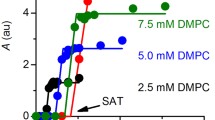
Styrene/maleic acid (SMA) and related copolymers are attracting great interest because they solubilise membrane proteins and lipids to form polymer-encapsulated nanodiscs. These nanodiscs retain a lipid-bilayer core surrounded by a polymer rim and can harbour a membrane protein or a membrane-protein complex. SMA exists in different styrene/maleic acid molar ratios, which results in differences in hydrophobicity and solubilisation properties. We have recently demonstrated fast collisional lipid transfer among nanodiscs encapsulated by the relatively hydrophobic copolymer SMA(3:1). Here, we used time-resolved Förster resonance energy transfer to quantify the lipid-transfer kinetics among nanodiscs bounded by SMA(2:1), a less hydrophobic copolymer that is superior in terms of lipid and membrane-protein solubilisation. Moreover, we assessed the effects of ionic strength and, thereby, the role of Coulombic repulsion in the exchange of lipid molecules among these polyanionic nanodiscs. Collisional lipid transfer was slower among SMA(2:1) nanodiscs (kcol = 5.9 M−1 s−1) than among SMA(3:1) nanodiscs (kcol = 222 M−1 s−1) but still two to three orders of magnitude faster than diffusional lipid exchange among protein-encapsulated nanodiscs or vesicles. Increasing ionic strength accelerated lipid transfer in a manner predicted by the Davies equation, an empirical extension of the Debye–Hückel limiting law, or an extended equation taking into account the finite size of the nanodiscs. Using the latter approach, quantitative agreement between experiment and theory was achieved for an effective nanodisc charge number of z ≈ –33, which is an order of magnitude less than their nominal overall charge.


Similar content being viewed by others
References
Aniansson EAG, Wall SN, Almgren M et al (1976) Theory of the kinetics of micellar equilibria and quantitative interpretation of chemical relaxation studies of micellar solutions of ionic surfactants. J Phys Chem 80:905–922
Bergethon PR (2010) The physical basis of biochemistry: the foundations of molecular biophysics, 2nd edn. Springer, London
Bersch B, Dörr JM, Hessel A et al (2017) Proton-detected solid-state NMR spectroscopy of a zinc diffusion facilitator protein in native nanodiscs. Angew Chemie Int Ed 56:2508–2512
Bjerrum N (1924) Zur Theorie der chemischen Reaktionsgeschwindigkeit. Z Phys Chem 108:82–100
Broecker J, Eger BT, Ernst OP (2017) Crystallogenesis of membrane proteins mediated by polymer-bounded lipid nanodiscs. Structure 25:1–9
Brønsted JN (1922) Zur Theorie der chemischen Reaktionsgeschwindigkeit. Z Phys Chem 102:169–207
Brønsted JN, Teeter CEJ (1923) On kinetic salt effect. J Phys Chem 28:579–587
Cuevas Arenas R, Danielczak B, Martel A et al (2017) Fast collisional lipid transfer among polymer-bounded nanodiscs. Sci Rep 7:45875
Cuevas Arenas R, Klingler J, Vargas C, Keller S (2016) Influence of lipid bilayer properties on nanodisc formation mediated by styrene/maleic acid copolymers. Nanoscale 8(32):15016–15026
Davies CW (1938) 397. The extent of dissociation of salts in water. Part VIII. An equation for the mean ionic activity coefficient of an electrolyte in water, and a revision of the dissociation constants of some sulphates. J Chem Soc 0:2093–2098.
Davies CW (1962) Ion association. Butterworths, Washington
Debye P, Hückel E (1923) Zur Theorie der Elektrolyte. I. Gefrierpunktserniedrigung und verwandte Erscheinungen. Phys Z 24:185–206
Dörr JM, Koorengevel MC, Schäfer M et al (2014) Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: the power of native nanodiscs. Proc Natl Acad Sci USA 111:18607–18612
Dörr JM, Scheidelaar S, Koorengevel MC et al (2016) The styrene–maleic acid copolymer: a versatile tool in membrane research. Eur Biophys J 45:3–21
Fullington DA, Nichols JW (1993) Kinetic analysis of phospholipid exchange between phosphatidylcholine/taurocholate mixed micelles: effect of the acyl chain moiety of the micellar phosphatidylcholine. Biochemistry 32:12678–12684
Fullington DA, Shoemaker DG, Nichols JW (1990) Characterization of phospholipid transfer between mixed phospholipid–bile salt micelles. Biochemistry 29:879–886
Grethen A, Oluwole AO, Danielczak B et al (2017) Thermodynamics of nanodisc formation mediated by styrene/maleic acid (2:1) copolymer. Sci Rep 7:11517
Guggenheim EA (1935) L. The specific thermodynamic properties of aqueous solutions of strong electrolytes. Phil Mag 19:588–643
Gulati S, Jamshad M, Knowles TJ et al (2014) Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochem J 461:269–278
Hazell G, Arnold T, Barker RD et al (2016) Evidence of lipid exchange in styrene maleic acid lipid particle (SMALP) nanodisc systems. Langmuir 32:11845–11853
Jones JD, Thompson TE (1990) Mechanism of spontaneous, concentration-dependent phospholipid transfer between bilayers. Biochemistry 29:1593–1600
Kemmer G, Keller S (2010) Nonlinear least-squares data fitting in Excel spreadsheets. Nat Protoc 5:267–281
Knowles TJ, Finka R, Smith C et al (2009) Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J Am Chem Soc 131:7484–7485
Kuriyan J, Konforti B, Wemmer D (2012) The molecules of life. Physical and chemical principles. Garland Science, New York
Lee SC, Knowles TJ, Postis VLG et al (2016) A method for detergent-free isolation of membrane proteins in their local lipid environment. Nat Protoc 11:1149–1162
Logez C, Damian M, Legros C et al (2016) Detergent-free isolation of functional G protein-coupled receptors into nanometric lipid particles. Biochemistry 55:38–48
Morrison KA, Akram A, Mathews A et al (2016) Membrane protein extraction and purification using styrene-maleic acid (SMA) copolymer: effect of variations in polymer structure. Biochem J 473:4349–4360
Nakano M, Fukuda M, Kudo T et al (2009) Static and dynamic properties of phospholipid bilayer nanodiscs. J Am Chem Soc 131:8308–8312
Nakano M, Fukuda M, Kudo T et al (2007) Determination of interbilayer and transbilayer lipid transfers by time-resolved small-angle neutron scattering. Phys Rev Lett 98:238101
Nichols JW (1985) Thermodynamics and kinetics of phospholipid monomer-vesicle interaction. Biochemistry 24:6390–6398
Nichols JW (1988) Phospholipid transfer between phosphatidylcholine-taurocholate mixed micelles. Biochemistry 27:3925–3931
Nichols JW, Pagano RE (1982) Use of resonance energy transfer to study the kinetics of amphiphile transfer between vesicles. Biochemistry 21:1720–1726
Nichols JW, Pagano RE (1981) Kinetics of soluble lipid monomer diffusion between vesicles. Biochemistry 20:2783–2789
Oluwole AO, Danielczak B, Meister A et al (2017) Solubilization of membrane proteins into functional lipid-bilayer nanodiscs using a diisobutylene/maleic acid copolymer. Angew Chemie Int Ed 56:1919–1924
Orwick-Rydmark M, Lovett JE, Graziadei A et al (2012) Detergent-free incorporation of a seven-transmembrane receptor protein into nanosized bilayer Lipodisq particles for functional and biophysical studies. Nano Lett 12:4687–4692
Orwick MC, Judge PJ, Procek J et al (2012) Detergent-free formation and physicochemical characterization of nanosized lipid-polymer complexes: Lipodisq. Angew Chemie Int Ed 51:4653–4657
Parmar M, Rawson S, Scarff CA et al (2017) Using a SMALP platform to determine a sub-nm single particle cryo-EM membrane protein structure. Biochim Biophys Acta 1860:378–383
Rharbi Y, Li M, Winnik MA, Hahn KG (2000) Temperature dependence of fusion and fragmentation kinetics of Triton X-100 micelles. J Am Chem Soc 122:6242–6251
Tonge SR, Tighe BJ (2001) Responsive hydrophobically associating polymers: a review of structure and properties. Adv Drug Deliv Rev 53:109–122
Vargas C, Cuevas Arenas R, Frotscher E, Keller S (2015) Nanoparticle self-assembly in mixtures of phospholipids with styrene/maleic acid copolymers or fluorinated surfactants. Nanoscale 7:20685–20696
Acknowledgements
Bartholomäus Danielczak (TUK) is gratefully thanked for helping with TR-FRET experiments. We thank Dr. Rodrigo Cuevas Arenas, Bartholomäus Danielczak, Erik Frotscher, Johannes Klingler, Florian Mahler, and Dr. Carolyn Vargas (all TUK) for fruitful discussions. This work was supported by the Carl Zeiss Foundation through the Centre for Lipidomics (CZSLip) and the Deutsche Forschungsgemeinschaft (DFG) through International Research Training Group (IRTG) 1830.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial or non-financial interest.
Rights and permissions
About this article
Cite this article
Grethen, A., Glueck, D. & Keller, S. Role of Coulombic Repulsion in Collisional Lipid Transfer Among SMA(2:1)-Bounded Nanodiscs. J Membrane Biol 251, 443–451 (2018). https://doi.org/10.1007/s00232-018-0024-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-018-0024-0