Abstract
Purpose
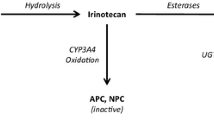
Irinotecan (CPT-11) is a drug used against a wide range of tumor types. The individualized dosing of CPT-11 is essential to ensure optimal pharmacotherapy in cancer patients, given the wide interindividual pharmacokinetic variability of this drug and its active metabolite SN-38. Moreover, the reabsorption from SN-38-G to SN-38, by enterohepatic recirculation, is critical due to its influence in the treatment tolerance. The aim of this research was to build a joint population pharmacokinetic model for CPT-11 and its metabolites (SN-38, and its glucuronide, SN-38-G) that enabled an individualized posology adjustment.
Methods
We used data of 53 treatment cycles of FOLFIRINOX scheme corresponding to 20 patients with metastatic colorectal cancer. In order to build the population pharmacokinetic model, we implemented parametric and non-parametric methods using the Pmetrics library package for R. We also built multivariate regression models to predict the area under the curve and the maximum concentration using basal covariates.
Results
The final model was a multicompartmental model which represented the transformations from CPT-11 to its active metabolite SN-38 and from SN-38 to inactive SN-38-G. Besides, the model also represented the extensive elimination of SN-38-G and the reconversion of the remaining SN-38-G to SN-38 by enterohepatic recirculation. We carried out internal validation with 1000 simulations. The regression models predicted the PK parameters with R squared adjusted up to 0.9499.
Conclusion
CPT-11, SN-38, and SN-38-G can be correctly described by the multicompartmental model presented in this work. As far as we know, it is the first time that a joint model for CPT-11, SN-38, and SN-38-G that includes the process of reconversion from SN-38-G to SN-38 is characterized.




Similar content being viewed by others
References
Quetglas EG, Armuzzi A, Wigge S, Fiorino G, Barnscheid L, Froelich M, Danese S (2015) Review article: the pharmacokinetics and pharmacodynamics of drugs used in inflammatory bowel disease treatment. Eur J Clin Pharmacol 71:773–799. https://doi.org/10.1007/s00228-015-1862-7
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécourarn Y, Adenis A, Raoul J-L, Gourgou-Bourgade S, de la Fochardiere C, Bennouna J, Bachet J-B, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Muranaka T, Kuwatani M, Komatsu Y, Sawada K, Nakatsumi H, Kawamoto Y, Yuki S, Kubota Y, Kubo K, Kawahata S, Kawakubo K, Kawakami H, Sakamoto N (2017) Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer. J Gastrointest Oncol 8:566–571. https://doi.org/10.21037/jgo.2017.02.02
Burris H, Fields S (1994) Topoisomerase I inhibitors. An overview of the camptothecin analogs. Hematol Oncol Clin North Am 8:333–355
Peterson C (2011) Drug therapy of cancer. Eur J Clin Pharmacol 67:437–447. https://doi.org/10.1007/s00228-011-1011-x
Berg AK, Buckner JC, Galanis E, Jaeckle KA, Ames MM, Reid JM (2015) Quantification of the impact of enzyme-inducing antiepileptic drugs on irinotecan pharmacokinetics and SN-38 exposure. J Clin Pharmacol 55:1303–1312. https://doi.org/10.1002/jcph.543
Poujol S, Bressolle F, Duffour J, Abderrahim AG, Astre C, Ychou M, Pinguet F (2006) Pharmacokinetics and pharmacodynamics of irinotecan and its metabolites from plasma and saliva data in patients with metastatic digestive cancer receiving Folfiri regimen. Cancer Chemother Pharmacol 58:292–305. https://doi.org/10.1007/s00280-005-0166-5
Valenzuela Jiménez B, González Sales M, Escudero Ortiz V, Martínez Navarro E, Pérez Ruixo C, Rebollo Liceaga J, González Manzano R, Pérez Ruixo JJ (2013) Influencia de los polimorfismos genéticos en UGT1A1, UGT1A7 and UGT1A9 sobre la farmacocinética de irinotecan, SN-38 y SN-38G. Farm Hosp 37:111–127. https://doi.org/10.7399/FH.2013.37.2.386
Wakefield J, Racine-Poon A (1995) An application of Bayesian population pharmacokinetic/pharmacodynamic models to dose recommendation. Stat Med 14:971–986. https://doi.org/10.1002/sim.4780140917
Asuphon O, Montakantikul P, Houngsaitong J, Kiratisin P, Sonthisombat P (2016) Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int J Infect Dis 50:23–29. https://doi.org/10.1016/j.ijid.2016.06.017
Oteo I, Lukas JC, Leal N, Suarez E, Valdivieso A, Gastaca M, Ortiz De Urbina J, Calvo R (2013) Tacrolimus pharmacokinetics in the early post-liver transplantation period and clinical applicability via Bayesian prediction. Eur J Clin Pharmacol 69:65–74. https://doi.org/10.1007/s00228-012-1300-z
Usman M, Frey OR, Hempel G (2017) Population pharmacokinetics of meropenem in elderly patients: dosing simulations based on renal function. Eur J Clin Pharmacol 73:333–342. https://doi.org/10.1007/s00228-016-2172-4
Oken M, Creech R, Tormey D, Horton J, Davis T, McFadden E, Carbone P (1982) Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 5:649–656
Castellanos Lácar MC (2003) Farmacocinética y farmacodinamia de irinotecan en pacientes con carcinoma colorrectal metastásico. Universidad de Navarra
Escoriaza J, Aldaz A, Castellanos C, Calvo E, Giráldez J (2000) Simple and rapid determination of irinotecan and its metabolite SN-38 in plasma by high-performance liquid-chromatography: application to clinical pharmacokinetic studies. J Chromatogr B 740:159–168
Jelliffe R, Schumitzky A, Van Guilder M, Wang X, Leary R (2001) Population pharmacokinetic models: parametric and nonparametric approaches In: 14th IEEE Symposium on Computer-Based Medical Systems (CBMS), pp 407–412
Leary R, Jelliffe R, Schumitzky A, Van Guilder M (2001) An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) population models. In: IEEE Symposium on Computer-Based Medical Systems pp 389–394
Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW (2012) Accurate detection of outliers and subpopulations with Pmetrics, a non-parametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. https://doi.org/10.1097/FTD.0b013e31825c4ba6
Chen S, Yueh M-F, Bigo C, Barbier O, Wang K, Karin M, Nguyen N, Tukey RH (2013) Intestinal glucuronidation protects against chemotherapy-induced toxicity by irinotecan (CPT-11). Proc Natl Acad Sci 110:19143–19148. https://doi.org/10.1073/pnas.1319123110
Xie R, Mathijssen RHJ, Sparreboom A, Verweij J, Karlsson MO (2002) Clinical pharmacokinetics of irinotecan and its metabolites: a population analysis. J Clin Oncol 20:3293–3301. https://doi.org/10.1200/JCO.2002.11.073
Klein CE, Gupta E, Reid JM, Atherton PJ, Sloan JA, Pitot HC, Ratain MJ, Kastrissios H (2002) Population pharmacokinetic model for irinotecan and two of its metabolites, SN-38 and SN-38 glucuronide. Clin Pharmacol Ther 72:638–647. https://doi.org/10.1067/mcp.2002.129502
Rosner GL, Panetta JC, Innocenti F, Ratain MJ (2008) Pharmacogenetic pathway analysis of irinotecan. Clin Pharmacol Ther 84:393–402. https://doi.org/10.1038/clpt.2008.63
Gupta E, Lestingi TM, Mick R, Ramirez J, Vokes EE, Ratain MJ (1994) Metabolic fate of irinotecan in humans: correlation of glucuronidation with diarrhea. Cancer Res 54:3723–3725
Mathjissen RHJ, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, Sparreboom A (2001) Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin Cancer Res 7:2182–2194. https://doi.org/10.1163/156856001300248353
Kodawara T, Higashi T, Negoro Y, Kamitani Y, Igarashi T, Watanabe K, Tsukamoto H, Yano R, Masada M, Iwasaki H, Nakamura T (2016) The inhibitory effect of ciprofloxacin on the β-Glucuronidase-mediated deconjugation of the irinotecan metabolite SN-38-G. Basic Clin Pharmacol Toxicol 118:333–337. https://doi.org/10.1111/bcpt.12511
Czejka M, Gruenberger B, Kiss A, Farkouh A, Schueller J (2010) Pharmacokinetics of irinotecan in combination with biweekly cetuximab in patients with advanced colorectal cancer. Anticancer Res 30:2355–2360
Satoh T, Yasui H, Muro K, Komatsu Y, Sameshima S, Yamaguchi K, Sugihara K (2013) Pharmacokinetic assessment of irinotecan, SN-38, and SN-38-glucuronide: a substudy of the FIRIS study. Anticancer Res 33:3845–3854
Innocenti F, Iyer L, Ratain MJ (2001) Pharmacogenetics of anticancer agents: lessons from amonafide and irinotecan. Drug Metab Dispos 29:596–600
Raymond E, Boige V, Faivre S, Sanderink GJ, Rixe O, Vernillet L, Jacques C, Gatineau M, Ducreux M, Armand JP (2002) Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. J Clin Oncol 20:4303–4312. https://doi.org/10.1200/JCO.2002.03.123
Rouits E, Charasson V, Pétain A, Boisdron-Celle M, Delord JP, Fonck M, Laurand A, Poirier AL, Morel A, Chatelut E, Robert J, Gamelin E (2008) Pharmacokinetic and pharmacogenetic determinants of the activity and toxicity of irinotecan in metastatic colorectal cancer patients. Br J Cancer 99:1239–1245. https://doi.org/10.1038/sj.bjc.6604673
Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, Elfring GL, Miller LL (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 343:905–914
Saltz LB, Douillard J-Y, Pirotta N, Alakl M, Gruia G, Awad L, Elfring GL, Locker PK, Miller LL (2001) Irinotecan plus fluorouracil/Leucovorin for metastatic colorectal cancer: a new survival standard. Oncologist 6:81–91
Woillard JB, Debord J, Monchaud C, Saint-Marcoux F, Marquet P (2017) Population pharmacokinetics and Bayesian estimators for refined dose adjustment of a new tacrolimus formulation in kidney and liver transplant patients. Clin Pharmacokinet 56:1491–1498. https://doi.org/10.1007/s40262-017-0533-5
Flint RB, ter Heine R, Spaans E, Burger DM, de Klerk JCA, Allegaert K, Knibbe CAJ, Simons SHP (2018) Simulation-based suggestions to improve ibuprofen dosing for patent ductus arteriosus in preterm newborns. Eur J Clin Pharmacol 74:1585–1591. https://doi.org/10.1007/s00228-018-2529-y
Kweekel D, Guchelaar HJ, Gelderblom H (2008) Clinical and pharmacogenetic factors associated with irinotecan toxicity. Cancer Treat Rev 34:656–669. https://doi.org/10.1016/j.ctrv.2008.05.002
Bozdogan H (1987) Model selection and Akaike’s Information Criterion (AIC): the general theory and its analytical extensions. Psychometrika 52:345–370. https://doi.org/10.1007/BF02294361
Funding
This work is partially supported by the “Ayuda para Doctorados Industriales del Ministerio de Economía, Industria y Competitividad” (Ref. DI-15-07511).
Author information
Authors and Affiliations
Contributions
AA conceived the study and contributed towards study design. EOI and AI analyzed the data. All authors were involved in the interpretation of data. EOI drafted the manuscript. OS and AA were involved in critical revision of the manuscript, with all study authors approving the final version for submission.
Corresponding author
Ethics declarations
This observational study was approved by the University Clinic of Navarre.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Methodology of non-compartmental analysis
A non-compartmental analysis was performed using the software WinNonlin version 8 with best fit approximation to the selection of the optimal regression line (which maximizes the coefficient of determination of linear regression) to estimate the elimination constant. We studied the following parameters:
-
AUCCPT-11: Area under the curve of CPT-11
-
AUCSN-38: Area under the curve of SN-38
-
AUCSN-38-G: Area under the curve of SN-38-G
-
C-MAXCPT-11: Maximum serum concentration that CPT-11 achieves
-
C-MAXSN-38: Maximum serum concentration that SN-38 achieves
-
C-MAXSN-38-G: Maximum serum concentration that SN-38-G achieves
-
T-MAXCPT-11: Time at which the C-MAXCPT-11 is observed
-
T-MAXSN-38: Time at which the C-MAXSN-38 is observed
-
T-MAXSN-38-G: Time at which the C-MAXSN-38-G is observed
-
IB: Biliary index [23],
-
IM: Metabolization index,
We examined the relationship between covariates and PK parameters resulting from the non-compartmental pharmacokinetic analysis using SPSS version 20 in order to delve into the repercussion of the physiopathological state in the pharmacokinetics of the treatment.
To that end, we used the Pearson or Spearman tests and scatter plots for continuous variables and Student’s t or Mann–Whitney U tests and boxplot diagrams for the categorical covariate (gender). The Levene test was used to check normality criteria. The significance level for all tests was set to 0.05 (P < 0.05).
We implemented, by means of a stepwise backward exclusion based on AIC [36], a multivariate regression for each PK parameter using those covariates that resulted statistically significant by the previous tests and the interactions among them. To select the model that best fitted the data, ANOVA test was used.
Non-compartmental pharmacokinetic results
The descriptive results of the parameters of non-compartmental pharmacokinetic analysis can be seen in Table 3. Within these PK parameters, some followed a normal distribution and others a non-normal distribution. AUCCPT-11, C-MAXCPT-11, C-MAXSN-38, C-MAXSN-38-G, and T-MAXCPT-11 followed a normal distribution and AUCSN-38, AUCSN-38-G, T-MAXSN-38, T-MAXSN.38-G, IB, and IM followed a non-normal distribution. Out of the 11 previous PK parameters, six showed correlations with the covariates presented in Table 1 and the dose. These are reflected in Table 4. In Figs. 5 and 6, different scatter plots with the PK individual parameters (AUC and C-MAX, respectively) are shown. The PK parameters are represented in the abscissa axis and the values of the covariates (in the cases where the correlation between them is significant at least at 0.05 level) are represented in the ordinate axis. With respect to the unique categorical covariate, there were no statistically significant differences in the mean/median between genders (Student’s t and Mann–Whitney U tests). In Fig. 7, we show boxplots of the PK parameters for each gender. The results of the multivariate regression analysis studying the relationships between covariates and PK parameters are shown in Table 5 for AUC values and in Table 6 for C-MAX values. The R squared adjusted (R2adj) value of the AUCCPT-11 regression was 0.6646 with 4.391 residual standard error (RSE), and the covariate dose and the interaction between ALP and neutrophils were statistically significant (P < 0.001 and P < 0.05, respectively). For the parameter AUCSN-38, R2adj was 0.2427 and RSE 0.1616, and in this case, the dose and LDH were statistically significant (P < 0.01). For AUCSN-38-G, R2adj was 0.9499 and RSE 0.4, and several covariates and interactions were statistically significant (see Table 5). For C-MAXCPT-11, R2adj was 0.5287 and RSE 0.6348, and dose and ALP were statistically significant (P < 0.001 and P < 0.01, respectively). For C-MAXSN-38, R2adj was 0.2862 and RSE 0.0197, and dose, TBil, and neutrophils were statistically significant (P < 0.05). Lastly, for C-MAXSN-38-G, R2adj was 0.4574 and RSE 0.0329, and dose, ALP, and the interaction between them were statistically significant (P < 0.001, P < 0.01, and P < 0.05, respectively).
Rights and permissions
About this article
Cite this article
Oyaga-Iriarte, E., Insausti, A., Sayar, O. et al. Population pharmacokinetic model of irinotecan and its metabolites in patients with metastatic colorectal cancer. Eur J Clin Pharmacol 75, 529–542 (2019). https://doi.org/10.1007/s00228-018-02609-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-02609-6