Abstract
Purpose
Valproic acid (VPA) is one of the most widely used antiepileptic drugs. Recently, increasing evidence suggested that polymorphisms in UGT2B7 gene were associated with VPA pharmacokinetics, but results remained controversial. Therefore, a meta-analysis was performed to derive a more precise evaluation between C802T, C161T, and G211T polymorphisms and plasma concentration of VPA.
Methods
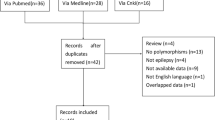
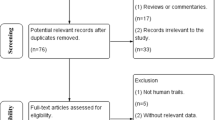
The PubMed, EMBASE, and the Cochrane library databases were searched for eligible studies. Articles meeting the inclusion criteria were comprehensively reviewed, and the available data were accumulated. The mean difference (MD) and 95% confidence interval (CI) were applied to assess the strength of the relationship.
Results
A total of 12 studies involving 1996 related East Asia epilepsy patients were assessed. We found that the UGT2B7 G211T polymorphism was associated with adjusted plasma VPA concentration (GG versus TT: P = 0.01, I 2 = 97%; GG versus GT: P < 0.00001, I 2 = 0%). Additionally, we also observed a significantly association between the C161T polymorphism and adjusted plasma VPA concentration (CC versus CT: P = 0.01, I 2 = 77%). Nevertheless, the pooled analysis showed that the C802T polymorphism had no significant effect on adjusted serum concentration of VPA.
Conclusions
The results of this meta-analysis demonstrated that UGT2B7 G211T and C161T polymorphisms were able to affect the pharmacokinetics in epilepsy patients treated with VPA, which provide further evidence for genetic effects of UGT2B7 gene on pharmacokinetics and pharmacodynamics of VPA. Epilepsy patients with these genotypes may be necessary to increase (or decrease) VPA dose to ensure its therapeutic effect.





Similar content being viewed by others
References
Göttlicher M (2004) Valproic acid: an old drug newly discovered as inhibitor of histone deacetylases. Ann Hematol 83:S91–S92
Jawień W, Wilimowska J, Kłys M, Piekoszewski W (2017) Population pharmacokinetic modelling of valproic acid and its selected metabolites in acute VPA poisoning. Pharmacol Rep 69(2):340–349. https://doi.org/10.1016/j.pharep.2016.12.003
Granneman GR, Wang SI, Machinist JM, Kesterson JW (1984) Aspects of the metabolism of valproic acid. Xenobiotica 14(5):375–387. https://doi.org/10.3109/00498258409151426
Wang C, Wang P, Yang LP, Pan J, Yang X, Ma HY (2017) Association of CYP2C9, CYP2A6, ACSM2A, and CPT1A gene polymorphisms with adverse effects of valproic acid in Chinese patients with epilepsy. Epilepsy Res 132:64–69. https://doi.org/10.1016/j.eplepsyres.2017.02.015
Guo Y, Hu C, He X, Qiu F, Zhao L (2012) Effects of UGT1A6, UGT2B7, and CYP2C9 genotypes on plasma concentrations of valproic acid in Chinese children with epilepsy. Drug Metab Pharmacokinet 27(5):536–542. https://doi.org/10.2133/dmpk.DMPK-11-NT-144
Ebner T, Burchell B (1993) Substrate specificities of two stably expressed human liver UDP-glucuronosyltransferases of the UGT1 gene family. Drug Metab Dispos 21(1):50–55
Du Z, Jiao Y, Shi L (2016) Association of UGT2B7 and UGT1A4 polymorphisms with serum concentration of antiepileptic drugs in children. Med Sci Monit 22:4107–4113. https://doi.org/10.12659/MSM.897626
Hung CC, Ho JL, Chang WL, Tai JJ, Hsieh TJ, Hsieh YW, Liou HH (2011) Association of genetic variants in six candidate genes with valproic acid therapy optimization. Pharmacogenomics 12(8):1107–1117. https://doi.org/10.2217/pgs.11.64
Coffman BL, King CD, Rios GR, Tephly TR (1998) The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y (268) and UGT2B7H (268). Drug Metab Dispos 26(1):73–77
Saito K, Moriya H, Sawaguchi T, Hayakawa T, Nakahara S, Goto A, Arimura Y, Imai K, Kurosawa N, Owada E, Miyamoto A (2006) Haplotype analysis of UDP-glucuronocyltransferase 2B7 gene (UGT2B7) polymorphisms in healthy Japanese subjects. Clin Biochem 39(3):303–308. https://doi.org/10.1016/j.clinbiochem.2006.01.002
Bhasker CR, McKinnon W, Stone A, Lo ACT, Kubota T, Ishizaki T, Miners JO (2000) Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics 10(8):679–685. https://doi.org/10.1097/00008571-200011000-00002
Sun Y, Zhuo W, Lin H et al (2015) The influence of UGT2B7 genotype on valproic acid pharmacokinetics in Chinese epilepsy patients. Epilepsy Res 114:78–80. https://doi.org/10.1016/j.eplepsyres.2015.04.015
Wang Q, Zhao L, Liang M, Dong Y, Yun W, Qiu F, Meng H, Guo Y (2016) Effects of UGT2B7 genetic polymorphisms on serum concentrations of valproic acid in Chinese children with epilepsy comedicated with lamotrigine. Ther Drug Monit 38(3):343–349. https://doi.org/10.1097/FTD.0000000000000271
Chu X, Zhang L, Wang G, Zhang S, Zhou J, Hao H (2012) Influence of UDP-glucuronosyltransferase polymorphisms on valproic acid pharmacokinetics in Chinese epilepsy patients. Eur J Clin Pharmacol 68(10):1395–1401. https://doi.org/10.1007/s00228-012-1277-7
Sun Y, Yu J, Yuan Q, Wu X, Hu J et al (2017) Early post-traumatic seizures are associated with valproic acid plasma concentrations and UGT1A6/CYP2C9 genetic polymorphisms in patients with severe traumatic brain injury. Scand J Trauma Resusc Emerg Med 25(1):85. https://doi.org/10.1186/s13049-017-0382-0
Wen Z, Fan S, Du C, Yin T, Zhou B et al (2016) Influence of acylpeptide hydrolase polymorphisms on valproic acid level in Chinese epilepsy patients. Pharmacogenomics 17(11):1219–1225. https://doi.org/10.2217/pgs-2016-0030
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Reny J, Combescure C, Daali Y et al (2012) Influence of the paraoxonase-1 Q192R genetic variant on clopidogrel responsiveness and recurrent cardiovascular events: a systematic review and meta-analysis. J Thromb Haemost 10(7):1242–1251. https://doi.org/10.1111/j.1538-7836.2012.04756.x
Chatzistefanidis D, Lazaros L, Giaka K, Nakou I, Tzoufi M, Georgiou I, Kyritsis A, Markoula S (2016) UGT1A6- and UGT2B7-related valproic acid pharmacogenomics according to age groups and total drug concentration levels. Pharmacogenomics 17(8):827–835. https://doi.org/10.2217/pgs-2016-0014
Inoue K, Suzuki E, Yazawa R, Yamamoto Y, Takahashi T, Takahashi Y, Imai K, Koyama S, Inoue Y, Tsuji D, Hayashi H, Itoh K (2014) Influence of uridine diphosphate glucuronosyltransferase 2B7-161C>T polymorphism on the concentration of valproic acid in pediatric epilepsy patients. Ther Drug Monit 36(3):406–409. https://doi.org/10.1097/FTD.0000000000000012
Ma H, Zhang T, Gong Z, Zhou B, Zou M, Xiao S, Zhu W (2013) Effect of UGT2B7 genetic variants on serum valproic acid concentration. Zhong Nan Da Xue Xue Bao Yi Xue Ban 38(8):766–772. https://doi.org/10.3969/j.issn.1672-7347.2013.08.002
Zhang H, Zhang W, Li Y et al (2017) Correlations between UGT2B7∗2 gene polymorphisms and plasma concentrations of carbamazepine and valproic acid in epilepsy patients. Brain Dev S0387-7604(17):30241–30243
Chen C, Xu T, Chen J, Zhou J, Yan Y, Lu Y, Wu S (2011) Allergy and risk of glioma: a meta-analysis. Eur J Neurol 18(3):387–395. https://doi.org/10.1111/j.1468-1331.2010.03187.x
Chadwick DW (1985) Concentration-effect relationships of valproic acid. Clin Pharmacokinet 10(2):155–163. https://doi.org/10.2165/00003088-198510020-00003
Huo T, Chen X, Lu X, Qu L, Liu Y, Cai S (2014) An effective assessment of valproate sodium-induced hepatotoxicity with UPLC-MS and (1)HNMR-based metabonomics approach. J Chromatogr B Analyt Technol Biomed Life Sci 969:109–116. https://doi.org/10.1016/j.jchromb.2014.08.011
Kiang TK, Ho PC, Anari MR, Tong V, Abbott FS, Chang TK (2006) Contribution of CYP2C9, CYP2A6, and CYP2B6 to valproic acid metabolism in hepatic microsomes from individuals with the CYP2C9*1/*1 genotype. Toxicol Sci 94(2):261–271. https://doi.org/10.1093/toxsci/kfl096
Holthe M, Rakvåg TN, Klepstad P, Idle JR, Kaasa S, Krokan HE, Skorpen F (2003) Sequence variations in the UDP-glucuronosyltransferase 2B7 (UGT2B7) gene: identification of 10 novel single nucleotide polymorphisms (SNPs) and analysis of their relevance to morphine glucuronidation in cancer patients. Pharmacogenomics J 3(1):17–26. https://doi.org/10.1038/sj.tpj.6500139
Sakaguchi K, Green M, Stock N, Reger TS, Zunic J, King C (2004) Glucuronidation of carboxylic acid containing compounds by UDP-glucuronosyltransferase isoforms. Arch Biochem Biophys 424(2):219–225. https://doi.org/10.1016/j.abb.2004.02.004
Innocenti F, Liu W, Fackenthal D, Ramírez J, Chen PX, Ye X, Wu X, Zhang W, Mirkov S, Das S, Cook E Jr, Ratain MJ (2008) Single nucleotide polymorphism discovery and functional assessment of variation in the UDP-glucuronosyltransferase 2B7 gene. Pharmacogenet Genomics 18(8):683–697. https://doi.org/10.1097/FPC.0b013e3283037fe4
Chung JY, Cho JY, Yu KS et al (2008) Pharmacokinetic and pharmacodynamic interaction of lorazepam and valproic acid in relation to UGT2B7 genetic polymorphism in healthy subjects. Clin Pharmacol Ther 83(4):595–600. https://doi.org/10.1038/sj.clpt.6100324
Liu L, Zhao L, Wang Q, Qiu F, Wu X, Ma Y (2015) Influence of valproic acid concentration and polymorphism of UGT1A4*3, UGT2B7-161C > T and UGT2B7*2 on serum concentration of lamotrigine in Chinese epileptic children. Eur J Clin Pharmacol 71(11):1341–1347. https://doi.org/10.1007/s00228-015-1925-9
Jin CJ, Miners JO, Lillywhite KJ, Mackenzie PI (1993) cDNA cloning and expression of two new members of the human liver UDP-glucuronosyltransferase 2B subfamily. Biochem Biophys Res Commun 194(1):496–503. https://doi.org/10.1006/bbrc.1993.1847
Sawyer MB, Innocenti F, Das S, Cheng C, Ramírez J, Pantle-Fisher FH, Wright C, Badner J, Pei D, Boyett JM, Cook E Jr, Ratain MJ (2003) A pharmacogenetic study of uridine diphosphate-glucuronosyltransferase 2B7 in patients receiving morphine. Clin Pharmacol Ther 73(6):566–574. https://doi.org/10.1016/S0009-9236(03)00053-5
Fattore C, Messina S, Battino D, Croci D, Mamoli D, Perucca E (2006) The influence of old age and enzyme inducing comedication on the pharmacokinetics of valproic acid at steady-state: a case-matched evaluation based on therapeutic drug monitoring data. Epilepsy Res 70(2–3):153–160. https://doi.org/10.1016/j.eplepsyres.2006.04.002
Perucca E, Grimaldi R, Gatti G, Pirracchio S, Crema F, Frigo GM (1984) Pharmacokinetics of valproic acid in the elderly. Br J Clin Pharmacol 17(6):665–669. https://doi.org/10.1111/j.1365-2125.1984.tb02401.x
Butler JM, Begg EJ (2008) Free drug metabolic clearance in elderly people. Clin Pharmacokinet 47(5):297–321. https://doi.org/10.2165/00003088-200847050-00002
Thibaudeau J, Lépine J, Tojcic J, Duguay Y, Pelletier G, Plante M, Brisson J, Têtu B, Jacob S, Perusse L, Bélanger A, Guillemette C (2006) Characterization of common UGT1A8, UGT1A9, and UGT2B7 variants with different capacities to inactivate mutagenic 4-hydroxylated metabolites of estradiol and estrone. Cancer Res 66(1):125–133. https://doi.org/10.1158/0008-5472.CAN-05-2857
Kiang TK, Ensom MH, Chang TK (2005) UDP-glucuronosyltransferases and clinical drug-drug interactions. Pharmacol Ther 106(1):97–132. https://doi.org/10.1016/j.pharmthera.2004.10.013
Funding
This work was supported by grants from the National Science Foundation of China (No. 81460560) and the Applied Basic Research Program of Yunnan Province (No. 2017FB134).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 50 kb)
Rights and permissions
About this article
Cite this article
Wang, P., Lin, XQ., Cai, WK. et al. Effect of UGT2B7 genotypes on plasma concentration of valproic acid: a meta-analysis. Eur J Clin Pharmacol 74, 433–442 (2018). https://doi.org/10.1007/s00228-017-2395-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2395-z