Abstract
Purpose
Therapeutic drug monitoring of patients receiving once daily aminoglycoside therapy can be performed using pharmacokinetic (PK) formulas or Bayesian calculations. While these methods produced comparable results, their performance has never been checked against full PK profiles. We performed a PK study in order to compare both methods and to determine the best time-points to estimate AUC0-24 and peak concentrations (C max).
Methods
We obtained full PK profiles in 14 patients receiving a once daily aminoglycoside therapy. PK parameters were calculated with PKSolver using non-compartmental methods. The calculated PK parameters were then compared with parameters estimated using an algorithm based on two serum concentrations (two-point method) or the software TCIWorks (Bayesian method).
Results
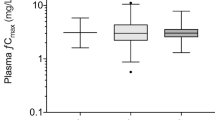
For tobramycin and gentamicin, AUC0-24 and C max could be reliably estimated using a first serum concentration obtained at 1 h and a second one between 8 and 10 h after start of the infusion. The two-point and the Bayesian method produced similar results. For amikacin, AUC0-24 could reliably be estimated by both methods. C max was underestimated by 10–20 % by the two-point method and by up to 30 % with a large variation by the Bayesian method.
Conclusions
The ideal time-points for therapeutic drug monitoring of once daily administered aminoglycosides are 1 h after start of a 30-min infusion for the first time-point and 8–10 h after start of the infusion for the second time-point. Duration of the infusion and accurate registration of the time-points of blood drawing are essential for obtaining precise predictions.



Similar content being viewed by others
References
Begg EJ, Barclay ML (1995) Aminoglycosides—50 years on. Br J Clin Pharmacol 39(6):597–603
Avent ML, Rogers BA, Cheng AC, Paterson DL (2011) Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J 41(6):441–449. doi:10.1111/j.1445-5994.2011.02452.x
Pagkalis S, Mantadakis E, Mavros MN, Ammari C, Falagas ME (2011) Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs 71(17):2277–2294. doi:10.2165/11597020-000000000-00000
Hustinx WN, Hoepelman IM (1993) Aminoglycoside dosage regimens. Is once a day enough? Clin Pharmacokinet 25(6):427–432. doi:10.2165/00003088-199325060-00002
Bartal C, Danon A, Schlaeffer F, Reisenberg K, Alkan M, Smoliakov R, Sidi A, Almog Y (2003) Pharmacokinetic dosing of aminoglycosides: a controlled trial. Am J Med 114(3):194–198. doi:10.1016/S0002-9343(02)01476-6
Barza M, Ioannidis JP, Cappelleri JC, Lau J (1996) Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ 312(7027):338–345. doi:10.1136/bmj.312.7027.33
Hatala R, Dinh TT, Cook DJ (1997) Single daily dosing of aminoglycosides in immunocompromised adults: a systematic review. Clin Infect Dis 24(5):810–815. doi:10.1093/clinids/24.5.810
Mavros MN, Polyzos KA, Rafailidis PI, Falagas ME (2011) Once versus multiple daily dosing of aminoglycosides for patients with febrile neutropenia: a systematic review and meta-analysis. J Antimicrob Chemother 66(2):251–259. doi:10.1093/jac/dkq451
Munckhof WJ, Grayson ML, Turnidge JD (1996) A meta-analysis of studies on the safety and efficacy of aminoglycosides given either once daily or as divided doses. J Antimicrob Chemother 37(4):645–663. doi:10.1093/jac/37.4.645
Turnidge J (2003) Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am 17(3):503–528. doi:10.1016/S0891-5520(03)00057-6
Bertino JS Jr, Booker LA, Franck PA, Jenkins PL, Franck KR, Nafziger AN (1993) Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 167(1):173–179. doi:10.1093/infdis/167.1.173
Croes S, Koop AH, van Gils SA, Neef C (2012) Efficacy, nephrotoxicity and ototoxicity of aminoglycosides, mathematically modelled for modelling-supported therapeutic drug monitoring. Eur J Pharm Sci 45(1–2):90–100. doi:10.1016/j.ejps.2011.10.022
Begg EJ, Barclay ML, Duffull SB (1995) A suggested approach to once-daily aminoglycoside dosing. Br J Clin Pharmacol 39(6):605–609. doi:10.1111/j.1365-2125.1995.tb05719.x
Begg EJ, Barclay ML, Kirkpatrick CM (2001) The therapeutic monitoring of antimicrobial agents. Br J Clin Pharmacol 52(Suppl 1):35S–43S. doi:10.1111/j.1365-2125.2001.00377.x
Barclay ML, Kirkpatrick CM, Begg EJ (1999) Once daily aminoglycoside therapy. Is it less toxic than multiple daily doses and how should it be monitored? Clin Pharmacokinet 36(2):89–98. doi:10.2165/00003088-199936020-00001
Coulthard KP, Peckham DG, Conway SP, Smith CA, Bell J, Turnidge J (2007) Therapeutic drug monitoring of once daily tobramycin in cystic fibrosis—caution with trough concentrations. J Cyst Fibros 6(2):125–130. doi:10.1016/j.jcf.2006.05.015
Barclay ML, Duffull SB, Begg EJ, Buttimore RC (1995) Experience of once-daily aminoglycoside dosing using a target area under the concentration–time curve. Aust N Z J Med 25(3):230–235. doi:10.1111/j.1445-5994.1995.tb01529.x
Burton ME, Brater DC, Chen PS, Day RB, Huber PJ, Vasko MR (1985) A Bayesian feedback method of aminoglycoside dosing. Clin Pharmacol Ther 37(3):349–357. doi:10.1038/clpt.1985.51
Wong C, Kumar SS, Graham GG, Begg EJ, Chin PK, Brett J, Ray JE, Marriott DJ, Williams KM, Day RO (2013) Comparing dose prediction software used to manage gentamicin dosing. Intern Med J 43(5):519–525. doi:10.1111/imj.12067
Zhang Y, Huo M, Zhou J, Xie S (2010) PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Prog Biomed 99(3):306–314. doi:10.1016/j.cmpb.2010.01.007
Sawchuk RJ, Zaske DE, Cipolle RJ, Wargin WA, Strate RG (1977) Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther 21(3):362–369
Fuchs A, Csajka C, Thoma Y, Buclin T, Widmer N (2013) Benchmarking therapeutic drug monitoring software: a review of available computer tools. Clin Pharmacokinet 52(1):9–22. doi:10.1007/s40262-012-0020-y
Acknowledgments
The authors would like to thank Claudia Bläsi, Luisa Baselgia-Jeker, and Beatrice Vetter for the excellent technical assistance.
Conflict of interest
None of the authors reports any conflict of interest with this work.
Funding source
This study was supported by a grant from the Swiss National Science Foundation to SK (SNF 31003A-132992).
Contribution of the authors
Lana Nezic: Prepared the first protocol, helped in the practical patient work, analyzed data, and approved the final version of the manuscript
Adrian Derungs: Helped in the practical patient work, analyzed data, helped to write the manuscript, and approved its final version
Marcel Bruggisser: Helped in preparing the final version of the protocol, helped in the practical patient work, and approved the final version of the manuscript
Sarah Tschudin-Sutter: Helped in preparing the final version of the protocol as an expert in infectiology and approved the final version of the manuscript
Stephan Krähenbühl: Approved the final version of the protocol, helped in writing the manuscript, and approved its final version
Manuel Haschke: Helped in preparing the final version of the protocol, analyzed data, helped in writing the manuscript, and approved its final version
Author information
Authors and Affiliations
Corresponding author
Additional information
Lana Nezic, Adrian Derungs, and Marcel Bruggisser contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(PDF 13 kb)
Supplementary Table 1
(PDF 13 kb)
Rights and permissions
About this article
Cite this article
Nezic, L., Derungs, A., Bruggisser, M. et al. Therapeutic drug monitoring of once daily aminoglycoside dosing: comparison of two methods and investigation of the optimal blood sampling strategy. Eur J Clin Pharmacol 70, 829–837 (2014). https://doi.org/10.1007/s00228-014-1680-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1680-3