Abstract
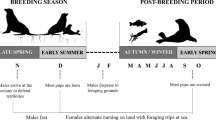
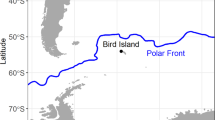
Individual variation in habitat and resource use has been reported for many top predators. This variation becomes important when comparing individuals taking into account sex, size, or age classes, since it can influence population dynamics and stability. We evaluated the individual variation and sexual/geographical isotopic niche overlap of the South American fur seal (SAFS) from the western South Atlantic. Whiskers of adult individuals from Brazil (n = 19), Uruguay (n = 29), and Argentina (n = 5) collected between 2005 and 2016 were serially sampled, resulting in 1001 samples, and their carbon and nitrogen isotopic ratios were analyzed longitudinally. According to its length, time integrated by whiskers ranged between 1.4 and 5.6 years. Males had δ13C (− 14.5 ± 0.6‰) and δ15N (18.9 ± 1.2‰) values significantly higher than females (δ13C = − 15.2 ± 0.5‰, δ15N = 17.8 ± 1.2‰). Females from Uruguay and Brazil were isotopically similar, displaying a large isotopic niche overlap (65.2–84%). Contrary, moderate isotopic niche overlaps were observed between males from Uruguay and Brazil (40.1–48.4%), and Uruguay and Patagonia (22.3–27.8%), indicating the use of different prey and/or feeding grounds. The WIC/TNW index of individual specialization pointed a significant specialization in males (0.38 for δ15N and 0.39 for δ13C). Females, on the other hand, are more generalists compared to males (0.53 and 0.71, for δ15N and δ13C, respectively). Differences in the ecological opportunity between sexes can account for these variations. Our study points out that trophic generalist populations of SAFS are composed of specialist and generalist individuals.


Similar content being viewed by others
References
Albernaz TL, Secchi ER, Oliveira LR, Botta S (2017) Ontogenetic and gender-related variation in the isotopic niche within and between three species of fur seals (genus Arctocephalus). Hydrobiologia 787:123–139
Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Lett 14:948–958
Araújo MS, Langerhans RB, Giery ST, Layman CA (2014) Ecosystem fragmentation drives increased diet variation in an endemic livebearing fish of the Bahamas. Ecol Evol 4:3298–3308
Barton K (2017) MuMIn: Multi-model inference. R package version 1.40.0. https://CRAN.R-project.org/package=MuMIn. Accessed 22 June 2017
Batallés LM, Pin O, Lima M (1990) Estudio del crecimiento del lobo fino sudamericano (Arctocephalus australis) en Isla de Lobos, Uruguay. Frente Marit 7:69–73
Baylis AM, Arnould JP, Staniland IJ (2014) Diet of South American fur seals at the Falkland Islands. Mar Mammal Sci 30:1210–1219
Bearhop S, Adams CE, Waldron S, Fuller RA, MacLeod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012
Bolnick DI, Yang LH, Fordyce JA, Davis JM, Svanbäck R (2002) Measuring individual-level resource specialization. Ecology 83:2936–2941
Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Botta S, Hohn AA, Macko SA, Secchi ER (2012) Isotopic variation in delphinids from the subtropical western South Atlantic. J Mar Assoc UK 92:1689–1698
Botto F, Gaitán E, Mianzan H, Acha M, Giberto D, Schiariti A, Iribarne O (2011) Origin and trophic pathways in a large SW Atlantic estuary: an evaluation using stable isotopes. Est Coast Shelf Sci 92:70–77
Braga ES, Chiozzini VC, Berbel GB, Maluf JC, Aguiar VM, Charo M, Eichler BB (2008) Nutrient distributions over the Southwestern South Atlantic continental shelf from Mar del Plata (Argentina) to Itajaí (Brazil): winter–summer aspects. Cont Shelf Res 28:1649–1661
Breed GA, Bowen WD, McMillan JI, Leonard ML (2006) Sexual segregation of seasonal foraging habitats in a non-migratory marine mammal. Proc R Soc Lond B Biol Sci 273:2316–2317
Bryan JE, Larkin PA (1972) Food specialization by individual trout. J Fish Board Can 29:1615–1624
Burnham KP, Anderson DR (2002) Model selection and multi-model inference: a practical information-theoretic approach. Springer, New York
Burone L, Ortega L, Franco-Fraguas P, Mahiques M, García-Rodriguez F, Venturini N, Marin Y, Brugnoli E, Nagai R, Muniz P, Bícego M, Figueira R, Salaroli A (2013) A multiproxy study between the Río de la Plata and the adjacente South-western Atlantic inner shelf to assess the sediment footprint of river vs. marine influence. Cont Shelf Res 55:141–154
Ceia FR, Ramos JA (2015) Individual specialization in the foraging and feeding strategies of seabirds: a review. Mar Biol 162:1923–1938
Cherel Y, Kernaléguen L, Richard P, Guinet C (2009) Whisker isotopic signature depicts migration patterns and multi-year intra-and inter-individual foraging strategies in fur seals. Biol Lett 5:830–832
Crawley MJ (2007) The R book. Imperial College London at Silwood Park, London
Crespo EA, Lewis MN, Campagna C (2007) Mamíferos marinos: pinnipedios y cetáceos. In: Carreto JI, Bremec C, Boschi EE (eds) El Mar Argentino y sus recursos pesqueiros 5. Editora Cidade, Buenos Aires, pp 127–150
Crespo EA, Schiavini A, García NA, Franco-Trecu V, Goodall RNP, Rodríguez D, Morgante JS, Oliveira LR (2015) Status, population trend and genetic structure of South American fur seals, Arctocephalus australis, in southwestern Atlantic waters. Mar Mammal Sci 31:866–890
de Mahiques MM, Tessler MG, Ciotti AM, da Silveira ICA, Sousa SHDM, Figueira RCL, Tassinari CCG, Furtado VV, Passos RF (2004) Hydrodynamically driven patterns of recent sedimentation in the shelf and upper slope off Southeast Brazil. Cont Shelf Res 24:1685–1697
DeNiro MJ, Epstein S (1976) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Di Tullio JC, Gandra TB, Zerbini AN, Secchi ER (2016) Diversity and distribution patterns of cetaceans in the subtropical southwestern Atlantic outer continental shelf and slope. PLoS One 11:e0155841
Drago M, Franco-Trecu V, Zenteno L, Szteren D, Crespo EA, Sapriza FR, Oliveira LR, Machado R, Inchausti P, Cardona L (2015) Sexual foraging segregation in South American sea lions increases during the pre-breeding period in the Río de la Plata plume. Mar Ecol Prog Ser 525:261–272
Fabiani A, Hoelzel AR, Galimberti F, Muelbert MM (2003) Long-range paternal gene flow in the southern elephant seal. Science 299:676
Franco-Trecu V (2017) Ecology and conservation status of the South American fur seal in Uruguay. In: Alava JJ (ed) Tropical pinnipeds. Bio-ecology, threats and conservation. CRC Press, Boca Raton
Franco-Trecu V, Aurioles-Gamboa D, Arim M, Lima M (2012) Prepartum and postpartum trophic segregation between sympatrically breeding female Arctocephalus australis and Otaria flavescens. J Mammal 93:514–521
Franco-Trecu V, Drago M, Riet-Sapriza FG, Parnell A, Frau R, Inchausti P (2013) Bias in diet determination: incorporating traditional methods in Bayesian mixing models. PLoS One 8:e80019
Franco-Trecu V, Aurioles-Gamboa D, Inchausti P (2014) Individual trophic specialisation and niche segregation explain the contrasting population trends of two sympatric otariids. Mar Biol 161:609–618
Garcia CA, Garcia VM (2008) Variability of chlorophyll-a from ocean color images in the La Plata continental shelf region. Cont Shelf Res 28:1568–1578
Graham BS, Koch PL, Newsome SD, McMahon KW, Aurioles D (2010) Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. In: West JB, Bowen GJ, Dawson TE, Tu KP (eds) Isoscapes—understanding movement, pattern, and process on Earth trough isotope mapping. Springer, Dordrecht, pp 299–318
Haimovici M (1998) Teleósteos demersais e bentonicos. In: Seeliger U, Odebrecht C, Castello JP (eds) Os ecossistemas costeiro e marinho do extremo sul do Brasil. Ecoscientia, Rio Grande, pp 143–151
Handley JM, Connan M, Baylis AM, Brickle P, Pistorius P (2017) Jack of all prey, master of some: influence of habitat on the feeding ecology of a diving marine predator. Mar Biol 164:82
Hirons AC, Schell DM, St. Aubin DJ (2001) Growth rates of vibrissae of harbor seals (Phoca vitulina) and Steller sea lions (Eumetopias jubatus). Can J Zool 79:1053–1061
Hutchinson GE (1957) Concluding remarks. Cold Spring Harb Symp Quant Biol 22:415–427
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602
Kernaléguen L, Cazelles B, Arnould JP, Richard P, Guinet C, Cherel Y (2012) Long-term species, sexual and individual variations in foraging strategies of fur seals revealed by stable isotopes in whiskers. PLoS One 7:e32916
Kernaléguen L, Arnould JP, Guinet C, Cherel Y (2015a) Determinants of individual foraging specialization in large marine vertebrates, the Antarctic and Subantarctic fur seals. J Anim Ecol 84:1081–1091
Kernaléguen L, Cherel Y, Knox TC, Baylis AM, Arnould JP (2015b) Sexual niche segregation and gender-specific individual specialisation in a highly dimorphic marine mammal. PLoS One 10:e0133018
Kernaléguen L, Cherel Y, Guinet C, Arnould JPY (2016) Mating success and body condition not related to foraging specializations in male fur seals. Open Sci 3:160143
Kiszka J, Oremus M, Richard P, Poole M, Ridoux V (2010) The use of stable isotope analyses from skin biopsy samples to assess trophic relationships of sympatric delphinids off Moorea (French Polynesia). J Exp Mar Biol Ecol 395:48–54
Kiszka J, Simon-Bouhet B, Martinez L, Pusineri C, Richard P, Ridoux V (2011) Ecological niche segregation within a community of sympatric dolphins around a tropical island. Mar Ecol Prog Ser 433:273–288
Layman CA, Araújo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR, Matich P, Rosenblatt AE, Vaudo JJ, Yeager LA, Post DM, Bearhop S (2012) Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol Rev 87:545–562
Lima M, Páez E (1995) Growth and reproductive patterns in the South American fur seal. J Mammal 76:1249–1255
Loizaga de Castro R, Saporiti F, Vales DG, García NA, Cardona L, Crespo EA (2015) Feeding ecology of dusky dolphins Lagenorhychus obscurus: evidence from stable isotopes. J Mammal 97:310–320
Mancini PL, Bugoni L (2014) Resources partitioning by seabirds and their relationship with other consumers at and around a small tropical archipelago. ICES J Mar Sci 71:2599–2607
McMahon KW, Hamady LL, Thorrold SR (2013) A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol Oceanogr 58:697–714
Müelbert MMC, Oliveira LR (2006) First records of stranded pregnant female South American fur seals, Arctocephalus australis, in the southern Brazilian coast. Lat Am J Aquat Mammal 5:67–68
Naya DE, Arim M, Vargas R (2002) Diet of South American fur seals (Arctocephalus australis) in Isla de Lobos, Uruguay. Mar Mammal Sci 18:734–745
Newsome SD, Tinker MT, Monson DH, Oftedal OT, Ralls K, Staedler MM, Foger ML, Estes JA (2009) Using stable isotopes to investigate individual diet specialization in California sea otters (Enhydra lutris nereis). Ecology 90:961–974
Newsome SD, Tinker MT, Gill VA, Hoyt ZN, Doroff A, Nichol L, Bodkin JL (2015) The interaction of intraspecific competition and habitat on individual diet specialization: a near range-wide examination of sea otters. Oecologia 178:45–59
Odebrecht C, Castello JP (2001) The convergence ecosystem in the Southwest Atlantic. In: Seeliger U, Kjerfve B (eds) Coastal marine ecosystems of Latin America. Springer, Berlin, pp 147–165
Oliveira LR (2004) Variação geográfica do lobo-marinho sul-americano, Arctocephalus australis (Zimmerman, 1783), com base em dados morfológicos e moleculares. Thesis, Universidade de São Paulo
Oliveira LR, Hoffman JI, Hingst-Zaher E, Majluf P, Muelbert MM, Morgante JS, Amos W (2008a) Morphological and genetic evidence for two evolutionarily significant units (ESUs) in the South American fur seal, Arctocephalus australis. Conserv Genet 9:1451–1466
Oliveira LD, Ott PH, Malabarba LR (2008b) Ecologia alimentar dos pinípedes do Sul do Brasil e uma avaliação de suas interações com atividades pesqueiras. In: Reis NR, Peracchi AL, Santos GASD (eds) Ecologia de Mamíferos. Technical Books, Londrina, pp 97–116
Pajuelo M, Bjorndal KA, Arendt MD, Foley AM, Schroeder BA, Witherington BE, Bolten AB (2016) Long-term resource use and foraging specialization in male loggerhead turtles. Mar Biol 163:235
Parnell AC, Phillips DL, Bearhop S, Semmens BX, Ward EJ, Moore JW, Jackson AL, Grey J, Kelly DJ, Inger R (2013) Bayesian stable isotope mixing models. Environmetrics 24:387–399
Peterson BJ (1999) Stable isotopes as tracers of organic matter input and transfer in benthic food webs: a review. Acta Oecol 20:479–487
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-Plus. Springer, New York, pp 3–56
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2017) nlme: Linear and nonlinear mixed effects models. R package version 3.1-131. https://CRAN.R-project.org/package=nlme. Accessed 22 June 2017
Piola AR, Matano RP (2001) Brazil and Falklands (Malvinas) currents. In: Steele JH, Thorpe SA, Turekian KK (eds) Ocean currents: a derivative of the encyclopedia of ocean sciences. Academic Press, San Diego, pp 35–43
Piola AR, Matano RP, Palma ED, Möller OO, Campos EJ (2005) The influence of the Plata River discharge on the western South Atlantic shelf. Geophys Res Lett 32:L01603
Ponce-de-León A (1984) Lactancia y composición cuantitativa de la leche del lobo fino sudamericano Arctocephalus australis (Zimmerman, 1783). ILPE: Industria Lobera y Pesquera del Estado, Montevideo, Uruguay. Anales 1:43–58
Ponce-de-León A (2000) Taxonomía, sistemática y sinopses de la biológia y ecologia de los pinipedios de Uruguay. In: Instituto Nacional de Pesca (INAPE), Ministerio de Ganadería, Agricultura y Pesca (MGAP) (eds) Sinopsis de la biologia y ecologia de las poblaciones de lobos finos y leones marinos de Uruguay. Pautas para su manejo y administración, Montevideo, pp 9–28
Prado JH, Mattos PH, Silva KG, Secchi ER (2016) Long-term seasonal and interannual patterns of marine mammal strandings in subtropical Western South Atlantic. PLoS One 11:e0146339
Riedman M (1990) The pinnipeds: seals, sea lions, and walruses (No. 12). University of California Press, Berkeley
Robertson A, McDonald RA, Delahay RJ, Kelly SD, Bearhop S (2015) Resource availability affects individual niche variation and its consequences in group-living European badgers Meles meles. Oecologia 178:31–43
Rosen DA (2009) Steller sea lions Eumetopias jubatus and nutritional stress: evidence from captive studies. Mammal Rev 39:284–306
Rossman S, Berens McCabe E, Barros NB, Gandhi H, Ostrom PH, Stricker CA, Wells RS (2015) Foraging habits in a generalist predator: sex and age influence habitat selection and resource use among bottlenose dolphins (Tursiops truncatus). Mar Mammal Sci 31:155–168
Roughgarden J (1974) Niche width: biogeographic patterns among Anolis lizard populations. Am Nat 108:429–442
Saporiti F, Bearhop S, Vales DG, Silva L, Zenteno L, Tavares M, Crespo EA, Cardona L (2015) Latitudinal changes in the structure of marine food webs in the Southwestern Atlantic Ocean. Mar Ecol Prog Ser 538:23–34
Sidorovich VE, MacDonald DW, Pikulik MM, Kruuk H (2001) Individual feeding specialization in the European mink, Mustela lutreola and the American mink, M. vison in north-esastern Belarus. Folia Zool 50:27–42
Stewart BS (1997) Ontogeny of differential migration and sexual segregation in northern elephant seals. J Mammal 78:1101–1116
Svendsen GM, Dans SL, González RA, Romero MA, Crespo EA (2013) Occurrence of South American fur seals Arctocephalus australis (Zimmermann, 1783) in San Matías Gulf, Patagonia, Argentina/Presencia del lobo marino de dos pelos Arctocephalus australis en el golfo San Matías, Patagonia, Argentina. Lat Am J Aquat Mammal 41:576–583
Syväranta J, Lensu A, Marjomäki TJ, Oksanen S, Jones RI (2013) An empirical evaluation of the utility of convex hull and standard ellipse areas for assessing population niche widths from stable isotope data. PLoS One 8:e56094
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 22 June 2017
Thompson D, Moss SE, Lovell P (2003) Foraging behaviour of South American fur seals Arctocephalus australis: extracting fine scale foraging behaviour from satellite tracks. Mar Ecol Prog Ser 260:285–296
Tyrrell LP, Newsome SD, Fogel ML, Viens M, Bowden R, Murray MJ (2013) Vibrissae growth rates and trophic discrimination factors in captive southern sea otters (Enhydra lutris nereis). J Mammal 94:331–338
Vales DG, Saporiti F, Cardona L, De Oliveira LR, Dos Santos RA, Secchi ER, Aguilar A, Crespo EA (2014) Intensive fishing has not forced dietary change in the South American fur seal Arctophoca (= Arctocephalus) australis off Río de la Plata and adjoining areas. Aquat Conserv Mar Freshw Ecosyst 24:745–759
Vales DG, Cardona L, García NA, Zenteno L, Crespo EA (2015) Ontogenetic dietary changes in male South American fur seals Arctocephalus australis in Patagonia. Mar Ecol Prog Ser 525:245–260
Van Valen L (1965) Morphological variation and width of ecological niche. Am Nat 99:377–390
Vander Zanden HB, Bjorndal KA, Bolten AB (2013) Temporal consistency and individual specialization in resource use by green turtles in successive life stages. Oecologia 173:767–777
Vaz-Ferreira R (1982) Arctocephalus australis (Zimmermann) South American fur seal. In: FAO mammals in the seas IV, Rome, pp 497–508
Vélez-Rubio GM, Cardona L, López-Mendilaharsu M, Souza GM, Carranza A, González-Paredes D, Tomás J (2016) Ontogenetic dietary changes of green turtles (Chelonia mydas) in the temperate southwestern Atlantic. Mar Biol 163:57
Wolf JBW, Kauermann G, Trillmich F (2005) Males in the shade: habitat use and sexual segregation in the Galápagos sea lion (Zalophus californianus wollebaeki). Behav Ecol Sociobiol 59:293–302
Zaccarelli N, Bolnick DI, Mancinelli G (2013) RInSp: an r package for the analysis of individual specialization in resource use. Methods Ecol Evol 4:1018–1023
Zeppelin TK, Orr AJ (2010) Stable isotope and scat analyses indicate diet and habitat partitioning in northern fur seals Callorhinus ursinus across the eastern Pacific. Mar Ecol Prog Ser 409:241–253
Zhao L, Schell DM (2004) Stable isotope ratios in harbor seal Phoca vitulina vibrissae: effects of growth patterns on ecological records. Mar Ecol Prog Ser 281:267–273
Acknowledgements
We would like to thank all researchers, students, and volunteers from Laboratório de Ecologia e Conservação da Megafauna Marinha/EcoMega-FURG who participated in data collection. Financial support was provided by ONG Yaqu Pacha and Comisión Sectorial Investigación Científica—UdelaR (Uruguay). National Council for Research and Technological Development (CNPq) provided a research fellowship to ERS (PQ 307846/2014-8). Our grateful thanks to Leandro Bugoni, Márcio Araújo, Danielle Monteiro, and the two anonymous reviewers for their helpful comments on the manuscript. Coordination for the Improvement of Higher Education Personnel (CAPES) provided a scholarship to RCL. This article is part of RC de Lima’s Master of Science Dissertation in Biological Oceanography (IO-FURG, RS, Brazil) under the supervision of SB and ERS, and is a contribution of the research group Ecologia e Conservação da Megafauna Marinha-EcoMega/CNPq.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DINARA (National Council for Aquatic Resources, Ministry of Livestock, Agriculture and Fishing, Uruguay) allowed access to the field sites and facilities during 2009 and 2010 (permits 572/2008 and 1022/2010). The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: T. L. Rogers.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by M. Sepulveda and an undisclosed expert.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Lima, R.C., Franco-Trecu, V., Vales, D.G. et al. Individual foraging specialization and sexual niche segregation in South American fur seals. Mar Biol 166, 32 (2019). https://doi.org/10.1007/s00227-019-3480-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3480-x