Abstract
Haploinsufficiency in SYNGAP1 is implicated in intellectual disability (ID) and autism spectrum disorder (ASD) and affects the maturation of dendritic spines. The abnormal spine development has been suggested to cause a disbalance of excitatory and inhibitory (E/I) neurotransmission at distinct developmental periods. In addition, E/I imbalances in Syngap1+/− mice might be due to abnormalities in K+–Cl− co-transporter function (NKCC1, KCC2), in a maner similar to the murine models of Fragile-X and Rett syndromes. To study whether an altered intracellular chloride ion concentration represents an underlying mechanism of modified function of GABAergic synapses in Dentate Gyrus Granule Cells of Syngap1+/− recordings were performed at different developmental stages of the mice. We observed depolarised neurons at P14–15 as illustrated by decreased Cl− reversal potential in Syngap1+/− mice. The KCC2 expression was decreased compared to Wild-type (WT) mice at P14–15. The GSK-3β inhibitor, 6-bromoindirubin-3ʹ-oxime (6BIO) that crosses the blood–brain barrier, was tested to restore the function of GABAergic synapses. We discovered that the intraperitoneal administration of 6BIO during the critical period or young adolescents [P30 to P80 (4-week to 10-week)] normalised an altered E/I balance, the deficits of synaptic plasticity, and behavioural performance like social novelty, anxiety, and memory of the Syngap1+/− mice. In summary, altered GABAergic function in Syngap1+/− mice is due to reduced KCC2 expression leading to an increase in the intracellular chloride concentration that can be counteracted by the 6BIO, which restored cognitive, emotional, and social symptoms by pharmacological intervention, particularly in adulthood.
Similar content being viewed by others
Introduction
Meticulous regulation of synaptic composition and function during the early stages of neuronal development through the molecular mechanisms of synaptic plasticity is fundamental for appropriate cognitive abilities in adulthood (Bliss and Collingridge 1993). Mutations in gene encoding proteins that regulate synaptic function disrupt this phenomenon and have been linked to the onset of neurodevelopmental brain-related disorders such as autism spectrum disorders (ASD) and Intellectual Disability (ID) (Bear et al. 2004; Penzes et al. 2011). One of the genes that have been implicated in ASD/ID is SYNGAP1 that encodes for RAS-GTPase-activating protein, SYNGAP1 (Hamdan et al. 2011; Berryer et al. 2013; Kilinc et al. 2018; Kimura et al. 2018; Vlaskamp et al. 2019). Neuropathophysiological hallmarks of the heterozygous Syngap1 mouse strain (Syngap1+/−) are enhanced excitatory neurotransmission, premature development of synapses, and an excitation/inhibition (E/I) imbalance. All these alterations during the development cause distortion of synaptic function and manifest abnormal cognitive and social behaviours in adulthood (Clement et al. 2012, 2013; Ozkan et al. 2014; Jeyabalan and Clement 2016; Muhia et al. 2010; Komiyama et al. 2002; Guo et al. 2009).
Syngap1+/− mice display improper glutamatergic synapse development and function; however, the trophic role of GABA in shaping synaptic function during development is unknown (Clement et al. 2012, 2013; Ozkan et al. 2014; Jeyabalan and Clement 2016; Kepecs and Fishell 2014; Ben-Ari 2002). Studies have shown that GABA exhibits trophic function—excitatory and inhibitory in early and late stages of development, respectively—regulated by potassium-chloride (Cl−) co-transporters like KCC2 and NKCC1. The NKCC1 expression level is age-dependently regulated and highest in the early stages of the development that allows influx of Cl−. In contrast, the KCC2 expression is high in the later stages of development, thereby, extrudes intracellular Cl− from the neuron. Hence, the intracellular Cl− concentration determines GABA-mediated responses that can further regulate the synaptogenesis and neuronal function during development (Ben-Ari 2002; Ben-Ari et al. 2012; Gamba 2005; Rivera et al. 1999, 2005; Blaesse et al. 2009). Alteration in the intracellular basal chloride concentration modulates the electrochemical gradient (EGABA) of the chloride ion flow through the chloride conductive GABAA receptor and thus affecting the strength of inhibitory action in a neuronal network. Thus, a switch between excitatory or inhibitory action of the GABAA receptor is mediated by the expression level of potassium-chloride-co-transporters. The disruption of the co-transporter expression during the critical period impairs synapse formation and function as it has been reported in several ID/ASD-like animal models (He et al. 2014; Hyde et al. 2011; Duarte et al. 2013; Deidda et al. 2015a, b). Besides, reversing the polarity of GABAA mediated chloride flow before the end of the critical period of mouse development by bumetanide corrected EGABA, synaptic function, and phenotypes in Down’s, Fragile -X and Rett syndrome-like animal models (He et al. 2019; Deidda et al. 2015a, b; Banerjee et al. 2016). Thus, normalisation of the reversal potential for chloride (here called GABA polarity) could represent a putative target for therapeutic intervention as GABAergic dysfunction, particularly during development, is one of the hallmarks of ID/ASD.
To restore reversal potentials of chloride GSK-3β (Glycogen synthase kinase-3, multifunctional serine/threonine kinase) has been suggested, which is pivotal in synaptic plasticity (Yao et al. 2002; Salcedo-Tello et al. 2011; Peineau et al. 2007; Liu et al. 2017). Increased activity of GSK-3β is a major contributor to the ID/ASD pathophysiology, and inhibiting GSK-3β reversed many of their phenotypes (Min et al. 2009; Mines and Jope 2011). Nevertheless, finding potent therapeutics that can target GSK-3β and restore phenotypes, especially when administered after the critical period of development. Therefore, the opportunity exists to develop a therapeutically viable GSK-3β inhibitor that can restore the phenotypes. We report a GSK-3β inhibitor, 6-bromoindirubin-3ʹ-oxime (6BIO), that has been described to cross the blood–brain barrier by LC-MSMS study and to be neuroprotective in an MPTP-based model of Parkinson’s disease (Meijer et al. 2003; Vougogiannopoulou and Skaltsounis 2012; Polychronopoulos et al. 2004; Suresh et al. 2017).
Given the minimal knowledge of GABAergic function during development in Syngap1+/−, we hypothesised that the GABA-mediated function regulated by chloride co-transporter expression and function is disrupted during development in Syngap1+/− mice. Therefore, the pharmacological intervention that modulates GABA function may restore phenotypes to a Wild-type level, particularly after the critical period of development.
Materials and methods
Experimental animals
C57/BL6 WT and Syngap1 Heterozygous Knock Out (B6; 129-Syngap1tm1Rlh/J, Syngap1+/−) mice were obtained from The Jackson Laboratory, USA, and inbred and maintained in the Institute’s animal house under 12 h dark and light cycle. Food and water were supplied ad libitum. All the experiments were performed in accordance with the Institutional Animal Ethics Committee (IAEC; Chair: Prof Anuranjan Anand) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). Female and male mice were studied in all experiments except in behaviour experiments where only male mice were observed. The experimenter was blind to the genotypes until the completion of the experiment.
Protein lysate preparation
Hippocampal lysates were prepared from Wild Type (WT) and Syngap1+/− littermates of (P: Postnatal day) P4, P7, P8, P14–15, P21–23, and P ≥ 90 in Phosphate Buffer Saline (PBS; pH 7.4). Homogenisation was done in 7 ml Dounce tissue homogenizer (Sigma, #D9063), containing RIPA Buffer (mM): 150 NaCl (Sigma, #S6191), 50 Tris–Cl, (Thermo Fischer Scientific, #15965), HCl (Merck Millipore, #HC301585), pH 7.4, 0.25% Sodium deoxycholate (Sigma Aldrich, #D6750), 5 EDTA (Merck Millipore, #324502), 0.10% SDS (BioRad, #1610302), 0.10% Triton X (HiMedia, #RM845), 10 µl/100 ml protease-inhibitor cocktail (Roche, #11836170001), 2 µl/100 ml phosphatase inhibitor cocktails (Sigma Aldrich, #P2850—cocktail 1, P5726—cocktail 2, #P0044—cocktail 3) in ice. After homogenisation, tissue was kept in ice for 30-min and further processed by centrifugation (Eppendorf, #5920R) at 4 °C at 16,000 g for 30-min. The supernatant was collected, aliquoted, and stored at − 80 °C (Panasonic, #MDF U55V-PE) until further use.
Immunoblot analysis
SDS-PAGE (Sodium dodecyl sulphate–Polyacrylamide gel electrophoresis (BioRad, # 1703812) and western blotting were performed by mixing samples with 5X dye (Tris–Cl pH 6.8, bromophenol blue dye (Thermo Fisher Scientific, #R0611), glycerol (Fischer scientific, #0854), DTT (di thioetriol) (HiMedia, #RM525) or 2-Mercaptoethanol (Himedia, #MB041). For NKCC1, samples were resolved in 5% stacking gel and 8% resolving gel and electrophoresed for approximately 3-h followed by wet transfer for 3-h at 80 V. Blocking was done in 5% skimmed milk (Himedia, #M530) for 1-h followed by incubation in anti-NKCC1 (Sigma Aldrich, #AV43805), and anti-β-ACTIN (Thermo Fisher Scientific, #GX2781) primary antibodies for 12-h followed by three washes with PBST (PBS and 1%Tween20 (Himedia, # GRM156) and 0.1% Tween20 (Himedia, # GRM156). Then, 1-h incubation in secondary antibody, Goat Anti-rabbit (BioRad, #1721019) in skimmed milk. After three washes with PBST, signals were visualised by the chemiluminescent method. For KCC2, the same procedure was followed except that samples were prepared in dye without DTT but in 3% 2-Mercaptoethanol, and resolved in 3–8% Tris–acetate gel (Thermo Fisher Scientific, #EA03752), and transferred overnight (16-h) (Blaesse et al. 2006). Blots were probed with anti-α-TUBULIN (CST, #2144S) and anti-KCC2 (Sigma Aldrich, #C2366) antibodies and visualised as mentioned earlier. The combined intensity of both the bands observed in the case of KCC2 after the chemiluminescent assay were considered for the analysis and plotted as a scatter plot with straight lines and markers (mean ± SEM).
Quantitative reverse transcriptase polymerase chain reaction (qPCR) analysis
mRNA expression levels of Nkcc1, Nkcc2, Kcc1, Kcc2, Kcc3, and Kcc4 were measured in male and female WT and Syngap1+/− from P14–15 mice. All primers procured from Eurofins and summarised in Table 1. Briefly, hippocampus samples were frozen immediately after dissection in liquid nitrogen (N2), kept in different vials, and stored at − 80 °C till homogenisation. RNA was prepared in 500 ml of Trizol (Sigma Aldrich, #T9424) at room temperature (RT) in 1 ml Dounce homogeniser (Wheaton, #357538), and 200 µl of CHCl3 (Fisher Scientific, # BP1145-1) was added and kept at RT for 5-min. The whole mix was centrifuged at 4 °C at 12,000 rpm for 20-min. The upper aqueous phase was transferred to a fresh tube, and 95% isopropanol (Sigma Aldrich, #59304) was added and incubated at RT for 1-h, followed by centrifugation at 4 °C at 10,000 rpm for 20-min. Pellet was washed with 70% ethanol (Merck Millipore, #100983) and centrifuged at 4 °C at 10,000 rpm for 10-min. The washing step was repeated twice, and the pellet was air-dried. Pellet was resuspended in 20 µl of nuclease-free water (Thermo Fisher Scientific, #AM9932), and RNA concentration was assessed in nanodrop. PCR was performed for cDNA synthesis by using a cDNA synthesis kit (Takara, #TP600) and aliquoted and stored at − 80 °C till further use. qPCR reaction was set up in duplicates using SYBR Mix (Roche, #light cycler 480) and probes for the genes mentioned above. Cq values were calculated by the ddCt method, in which dCt was calculated by subtracting test gene value from housekeeping gene value and then taking 2^-dCt as Cq value. The relative mRNA level, 2-ddCt, was plotted.
Cryosectioning and immunofluorescence (IF)
For immunohistochemical analysis, P8, P14–15, and P ≥ 90 male and female mice were anaesthetised with halothane (Alem, #M14A) and perfused with ice-chilled 150 ml PBS followed by 150 ml of 4% paraformaldehyde (PFA) (Sigma Aldrich, #158127) ~ 50 ml/minute using a peristaltic pump (Ravel Hiteks #RH-P110S-25). The brain was dissected out and incubated in 4% PFA overnight, followed by incubation in 30% sucrose (Sigma Aldrich, #S9378) till it submerged to the bottom and stored at − 80 °C until further use. The brain was kept in Optimum Cutting Temperature (OCT) solution (Trivitron, #DIG-46181) and incubated for 20-min inside cryotome at − 20 °C (Leica, #CM3050). 30 µm sections were obtained and stored in 1 X PBS. Antigen retrieval was performed by treating sections with Sodium citrate buffer (Fisher Scientific, #27625), pH 6.0 for 30-min followed by three washes, 10-min each, by 0.1% PBSTx [0.1% Triton X 100 (Himedia #RM845) in PBS] and then blocking [2% BSA (Himedia, #GRM105) + 1% Goat Serum (MP Bio, #0219135680) + 0.1% PBSTx incubation for 4-h at RT]. Sections were given three washes with 0.1% PBSTx for 10-min each and then incubated in anti-KCC2 and anti-SYNGAP1 antibody for 2-h at RT and 34-h at 4 °C followed by three washes with 0.1% PBSTx and kept in secondary antibody (Thermo Fisher Scientific, Alexa Flour 633 goat anti-mouse, #A21052 and Alexa Flour 555 goat anti-rabbit, #A21428) for 4-h at RT. Three washes followed secondary incubation with 0.1% PBSTx and then incubation in Hoechst (Thermo Fisher Scientific, #H3570) for 10-min followed by a wash of 10-min with PBS. Sections were mounted in Vectashield (Vector, #H-1000) on slides. The imaging was performed in a confocal laser scanning microscope (LSM 880, Zeiss, India) and analysed using ImageJ software. Dentate gyrus granular cells (DGGC) were chosen for imaging, and all the images were acquired with a confocal-fluorescence microscope equipped with a 63X objective. A Z-stacks of 30 images were acquired, and images #10 to #24 were considered for analysis. The maximum intensity projection (MIP) image over 15 confocal images of a Z-stack was calculated and analysed. Image thresholds were kept constant to quantify the expression of KCC2 for all genotypes and age groups. The integrated density of the isolated areas was calculated to measure the mean intensity (Madhwal et al. 2020; Shim et al. 2012). In representative images (MIP images), punctate structures indicate expression of KCC2 (red colour) and SYNGAP1 (green colour) on the YFP background (yellow colour). Thy1-Yfp-Syngap1+/− mice were used to identify the labelling of excitatory neurons. The whole area of the image represented along with YFP expressing neurons was considered for the analysis. The analysis of genotypes was performed in parallel, and the statistical comparison between groups was evaluated with the Unpaired Student’s t-test.
Preparation of hippocampal slices
Acute brain slices were prepared from WT and Syngap1+/− mice from P4–5, P7, P8, P14–15, P21–23, and P ≥ 90 sacrificed by cervical dislocation. Following decapitation, the brain was removed and kept in an ice-cold (almost frozen) cutting solution, containing (mM),: 189 Sucrose (Sigma Aldrich, #S9378), 10 d-Glucose (Sigma Aldrich, #G8270), 26 NaHCO3 (Sigma Aldrich, #S5761), 3 KCl (Sigma Aldrich, #P5405), 10 MgSO4.7H2O (Sigma Aldrich, #M2773), 1.25 NaH2PO4 (Sigma Aldrich, #S8282), and 0.1 CaCl2 (Sigma Aldrich, #21115). After 30-s, the brain was glued to the brain holder of a vibratome (Leica, #VT1200S), 350 µm thick slices were prepared, and the cortex was dissected to isolate the hippocampus. All the slices were kept at 37 °C in a water bath (Thermo Fisher Scientific, #2842) for 50-min in a slice chamber containing aCSF (artificial cerebrospinal fluid), composed of (mM): 124 NaCl (Sigma Aldrich, #S6191), 3 KCl, 1 MgSO4.7H2O, 1.25 NaH2PO4, 10 D-Glucose, 24 NaHCO3 and 2 CaCl2 bubbled with carbogen (5% CO2 and 95% O2; Chemix, India). The osmolarity of the sucrose-based cutting solution and regular aCSF were in the range of 278–285 mOsm/kg based on the reading from the osmometer (Advanced Instruments Inc, USA, Osmometer Model 3320), and pH was 7.4. We checked the osmolarity of all the solutions, including the internal solution (280–290 mOsm/kg, pH 7.3), whenever it was prepared fresh. Following recovery, slices were maintained at RT until transferred to a submerged chamber of ~ 1.5 ml volume in which the slices were perfused continuously (2–3 ml/minute) with warmed (34 °C) and carbogenated aCSF.
Perforated and whole-cell patch-clamp recordings
The reversal potential of GABAAR (EGABA) was measured using Gramicidin (Sigma Aldrich, #G5002) based perforated patch-clamp of DGGC (Dentate Gyrus Granule Cells) from WT and Syngap1+/− littermates at P4–5, P7, P8, P14–15, P21–23, and P ≥ 90. 5–6 MΩ glass electrode (ID: 0.69 mm, OD: 1.2 mm, Harvard Apparatus) tip was filled with an internal solution containing (mM): 130 K-Gluconate (Sigma Aldrich, #P1847), 20 KCl (Sigma Aldrich, #P5405), 10 HEPES free acid (OmniPur Merck Millipore, #5310), 0.2 EGTA (OmniPur Merck Millipore, #4100), ATP and GTP, then backfilled with 100 μM Gramicidin. GABAR-mediated currents were evoked using a bipolar stimulating electrode (CBARC75, FHC, USA) connected to a constant current stimulator (Digitimer, UK) and by holding the cell at different membrane potentials ranging from − 100 to + 40 mV in the presence of AMPAR and NMDAR blockers, 10 μM 6-Cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) (Tocris, #1045) and 100 μM (2R)-amino-5-phosphonovaleric acid (DL-AP5) (Tocris, #3693) respectively. Whole-cell recordings were performed to measure giant inward currents (GICs), GABA-mediated activities, and tonic currents mediated by GABARs by holding the neurons in DGGC at − 70 mV. In the case of GICs and GABA-mediated activities (at P14–16), K-Gluconate based internal solution (composition is the same as described earlier) was used, and 10 μM Bicuculline (Bic) was bath applied after recording 10-min of baseline and GICs were recorded for 5-min. Tonic currents were measured using CsCl based internal solution containing (mM): 120 CsCl (Sigma Aldrich, #289329), 10 HEPES free acid, 10 EGTA, 4 MgCl2 (Sigma Aldrich, #M8266), 5 Lidocaine N-ethyl bromide (Sigma Aldrich, #5783), ATP and GTP. To isolate GABA-mediated currents, we bath applied AP-5 (100 μM) and CNQX (10 μM) for 10-min followed by NO-711 hydrochloride (1–2-(Diphenylmethylene)iminooxyethyl-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid hydrochloride; GAT-1 inhibitor to increase the ambient GABA concentration; 20 μM was used; Sigma Aldrich, #N142) for 10-min, and then tonic currents were blocked by Bicuculine (20 μM) (Cope et al. 2009; Nusser and Mody 2002). An increase in holding current after the application of Bicuculine was calculated and plotted against current amplitude in the presence or absence of Bicuculine. Cells with internal solution based on K-Gluconate (GICs, GABA-mediated activity, and chloride reversal potential experiments), showing a holding current of < − 30 pA were discarded from the analysis. Cells with internal solution based on CsCl (GABA-mediated tonic currents experiment) showing a holding current of < − 85 pA were discarded from the analysis. The data are based on experiments with at least three mice (N) or more and slices of 5 or more (n) per group.
Extracellular field recordings
Field excitatory postsynaptic potential (fEPSP) were elicited by placing a concentric bipolar stimulating electrode connected to a constant current isolator stimulator unit at Schaffer-collateral commissural pathway and recorded with a 3–5 MΩ resistance glass pipette filled with aCSF. Signals were amplified using an Axon Multiclamp 700B amplifier (Molecular Devices), digitised using an Axon Digidata 1440A (Molecular Devices), and stored on a computer using pClamp10.7 software (Molecular Devices). Stimulation frequency was set at 0.05 Hz. A baseline period of 15-min fEPSP was recorded at a stimulation intensity that elicited an approximately half-maximal response. Following the baseline period, the theta-burst stimulation (TBS) was delivered consisting of five bursts at 5 Hz (theta frequency), repeated four times at an interval of 20 s (Booth et al. 2014). fEPSPs after TBS were recorded for 45 min. Slices with a high fibre volley FV/fEPSP ratio and unstable baseline were discarded from the analysis. The exclusion threshold was considered as when the ratio of the amplitude of FV/fEPSP was more than 1:3. Data analysis was performed using Clampfit 10.7 and Excel 2019. Two-Way ANOVA was performed to determine the statistical significance between the last 3-min of baseline and post-LTP. Example traces are those recorded for 5-min around the time point indicated in the graph.
Behavioural studies
In the 6BIO study, the mice in the drug group were administered intraperitoneally with 5 mg/kg of 6BIO. All four experimental groups of mice of three different age groups were injected daily (vehicle or 6BIO) as per the age group and on the day of behavioural or recording experiments an hour before the commencement of the experiment. The injection was done once a 24-h and time (~ 9 AM), either on normal days or the day of the experiment, was kept the same. Mice were kept in the behaviour room for at least 20-min before behaviour experiments. The light intensity was maintained at 90–110 LUX. The experimenter who analysed the data was blind to the experimental groups.
Open-field test (OFT)
OFT was conducted in a 52 × 50 × 45 cm custom-made white plywood wooden box in JNCASR. Mice were left at the periphery in one of the corners of the box and allowed to explore the arena for 15 min. Before introducing other mice, the arena was cleaned with 70% ethanol and left for 5-min air dry. The activity of the mice was recorded with SMART v3.0.04 (Panlab Harvard Apparatus #DCBA5-E83) and a video camera (SONY #SSC-G118). Total distance travelled and the number of entries to the centre was determined by SMART v3.0.04 (Panlab Harvard Apparatus #DCBA5-E83).
Novel object recognition (NOR)
The NOR was performed in a custom-made wooden box 35 × 35 × 35 cm in JNCASR. On Day-1, mice were familiarised with the arena without the objects for 5-min. Day-2 and Day-3 sessions included habituation with two familiar objects placed diagonally to each other, and mice were allowed to explore the objects for 10 min. The day-4 session involved the recognition memory test 24 h after the last training. The time the mice explored the novel object and a familiar object placed diagonally was taken within 10 min (Lueptow 2017; Leger et al. 2013). The time of interaction of the mice (sniffing/touch) to familiar and novel objects was acquired manually using stopwatches. The discrimination index (DI) for novel object recognition test (NOR) [(time spent with novel object—time spent with familiar object)/(time spent with novel object + time spent with familiar object)] × 100, was calculated and taken as a measure of the strength of object recognition (either novel or familiar). DI values above 25 correlated with memory, and below 25 correlated with a lack of memory. In addition, the mice behaviour was monitored and protocolled by a Handycam (SONY #HDR-CX405).
Social interaction (SI) and social preference (SP)
SI and SP were performed in a custom made transparent three-chamber plexiglass box 40.6 × 21 × 35 cm in JNCASR. In the first session of the experiment, the mouse was habituated to the three-chambered glass box for 5-min. In the second session, the chambers were closed with doors, and the mouse was placed in the middle chamber and allowed to explore for 5-min. In the third session, two steel mesh jars were kept in either of the adjacent chambers—one had a mouse of the same gender and age, and the other was empty. The test mouse was introduced into the middle chamber, the doors were removed, and the mouse explored the whole arena for 10-min (Nakajima et al. 2019). Social interaction was calculated by the time spent between the empty jar and familiar mice. In the last session, the mouse of the same gender and age was introduced into the empty mesh jar, and the test mouse was allowed to explore and interact with both the mice placed beneath the mesh jars. Social preference was calculated by the time spent between familiar and unfamiliar mice. The time mouse spent climbing over the mesh jar was not taken into consideration in the analysis. SI and SP were recorded using Handycam (SONY #HDR-CX405).
Flurothyl-induced seizure
Seizure testing was performed in a chemical fume hood. Mice were kept in the test room for 30-min prior to the start of the experiment. Mice were placed in the closed cylindrical transparent box inserted with the narrow tube whose outlet was onto the filter paper and was attached to the Syringe pump (New era pump system #NE-1000). Seizures were induced using 10% flurothyl (Sigma Aldrich #287571) and 95% ethanol (Merck #K46475383 511) as solvent at the rate of 200 μl/min using a Syringe pump. The infusion was stopped after the onset of hind limb extension. The seizure threshold was measured and plotted as the latency (seconds) from the beginning of the infused flurothyl drop on the filter paper to the generalised tonic–clonic seizure (rhythmic jerky movement at a higher pace with the loss of postural control). Duration of seizure was calculated as the time of the start of the generalised tonic–clonic seizure to the time when the mice regained its posture and stopped showing signs of clonus or jerky movement of limbs and body, in general (Kadiyala et al. 2014; Samoriski and Applegate 1997; Ferland 2017). The video was recorded using Handycam (SONY #HDR-CX405), analysis was done manually. The intensity was measured based on the grade of seizure occurrence and plotted as a score. For example, Grade 5 was considered for tonic–clonic seizure. Hence, the score given was 5. We have provided the table for the reference of Grade and Score (Table 2) considered for the current study. We choose a 10-min window in which we have observed, upon flurothyl infusion, how many deaths occurred in all four groups. If mice did not die in that window, we gave them a 5 for the intensity analysis. We have followed Ferland R. J. paper for determining the grade of seizure, with slight modification (Ferland 2017).
Statistics
All graphs were plotted, and statistical analysis was performed in Microsoft Excel (2019) and Prism GraphPad (version 8.3). Data distributions were not tested for normality using specific statistical tests as we found that the residual values for ANOVA were very less. Data points having more than three standard deviation differences were excluded from the analysis, where ANOVA has been performed. To verify these results, Grubb’s test (GraphPad) was used and further verified using the Interquartile Range. Data are presented as Mean ± Standard Error of Mean (SEM) except for box and whisker plots. Unpaired Student’s t-test assessed the genotype difference for a given age group while Two-way ANOVA followed Tukey’s post-hoc to determine genotype difference across other age groups unless otherwise mentioned.
Results
Weakened neuronal network activity and inhibition at 2 weeks in Syngap1 +/−
We asked whether E/I imbalance during development a consequence of GABA-mediated dysfunction at the neuronal circuit level is by measuring giant inward currents (GICs) and GABA-mediated activity from dentate gyrus granular cells (DGGC) (Ben-Ari et al. 1989; Griguoli and Cherubini 2017; Sipila et al. 2005). We have mentioned “GABA-mediated activity” in our data as GICs does not occur after P12 (Ben-Ari et al. 1989) as our main question was to understand whether GABA still contributes to the excitability of neurons as we have observed changes in the function of GABA (Fig. 1C). Thus we plotted the number of events (interictal discharges) represented as GABA-mediated activity in the presence of Bicuculline (cell held at − 70 mV and baseline was performed for 10-min in aCSF before adding Bicuculline). It is an indirect measure of GABA-mediated events as Bicuculline is a GABAA blocker. The GABA-mediated activity was measured at P14 as maximum disruption of synaptic function occurred in Syngap1+/ mice at this age (Clement et al. 2012). In the absence of 10 µm bicuculline, no significant differences in GIC were observed between genotypes. However, in the presence of Bicuculline, the number of GICs and GABA-mediated activity decreased significantly in comparison with before drug application at P8–9 (Supplementary Fig. 1A, E) and P14–15 (Supplementary Fig. 1B, F), respectively, in Syngap1+/ mice. A single GIC with expanded timescale suggests GIC as network events of 300–500 pA amplitude that lasts ~ 300 ms (Supplementary Fig. 1C, D), without the involvement of an action potential. No statistically significant difference was observed between with and without Bicuculline at PND14-16 in the WT. The experiments suggest that GABA is excitatory in Syngap1+/−. Disrupted activity within the DGGC could be due to impaired GABA-mediated tonic currents at P14–15. Using whole-cell patch-clamp from DGGC, we observed a significant GABA-mediated holding current component in WT (Wild Type) but not in Syngap1+/− (Supplementary Fig. 1G, H). Thus, a diminished strength of tonic inhibition due to altered GABA-mediated activity may contribute to increased excitability during development in Syngap1+/− (Clement et al. 2012, 2013; Ozkan et al. 2014).
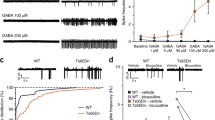
Neurons of Syngap1+/− mice show a shift in chloride reversal potential (ECl−) at P14–15. A Left, Evoked representative Cl− mediated current traces at the indicated holding potentials of a Gramicidin-based perforated voltage-clamp experiment. Right, The graph to the right summarises the relationship of holding potential (mV) to current amplitude for one representative experiment and depicts the trend as a linear fit curve. The experiments were conducted at P14–15 in WT mice. B Same as A but in P14–15 Syngap1+/−. C The graph summarises the reversal potentials of GABA receptor-mediated Cl− current (ECl−) in WT as well as Syngap1+/− at the indicated postnatal ages. P4-5: WT: N = 6, n = 7, 35 ± 1.37; Syngap1+/−: N = 4, n = 7, 48 ± 3, (NS: p > 0.05); P7: WT: N = 3, n = 4, 44 ± 2.78; Syngap1+/− N = 3, n = 5, 47 ± 3, (NS: p > 0.05); P8: WT N = 5, n = 6, 46 ± 1; Syngap1+/−: N = 4, n = 8, 51 ± 4.04, NS: p > 0.05; P14–15: WT: N = 3, n = 7; Syngap1+/−: N = 4, n = 6, ***p < 0.001; P21–23 WT, N = 4, n = 6, 65 ± 2; Syngap1+/−: N = 3, n = 7, 64 ± 2, NS: p > 0.05; P ≥ 90: WT: N = 7, n = 15, 68 ± 1.53; Syngap1+/−, N = 5, n = 9, 71 ± 2.18, NS: p > 0.05. F(5, 76) = 6.83, p < 0.0001 for the interaction. Data are presented as single data points and means ± SEM. N number of mice, n number of cells. Two-way ANOVA Tukey’s multiple comparisons test
Disrupted GABA polarity switch in Syngap1 +/− mice at P14–15
Dysfunctions of GABA-mediated effects on neuronal networks might be the result of an altered GABA reversal potential (ECl−). GABA-mediated synaptic responses were elicited by stimulating the medial perforant pathway (MPP) in the presence of NMDAR and AMPAR blockers. Perforated whole-cell voltage-clamp recordings were conducted on DGGCs of WT and Syngap1+/− mice at different developmental stages. The neurons were voltage-clamped between − 100 to + 40 mV to measure chloride reversal potentials (Fig. 1A, B). Analyses revealed a reduced ECl− in DGGCs of P14-15-old Syngap1+/− mice (Fig. 1C). A reduction in ECl− corresponds to the excitatory action of GABA-mediated synaptic transmission in Syngap1+/−. The altered ECl− of 2-week-old Syngap1+/− mice will alter the direction of chloride flow, leading to reduced membrane potential and to GABA-mediated excitatory synaptic signals in the early stages of development, leading to altered E/I.
Altered expression of NKCC1 and KCC2 during the development of Syngap1 +/− mice
NKCC1 and KCC2 regulate chloride homeostasis and excitatory-to-inhibitory shift of GABA during the development. Therefore, it may indirectly regulate the E/I balance in the brain, which is known to be compromised in several neurodevelopmental disorders (Blaesse et al. 2009; Ben-Ari et al. 2012; Gamba 2005; He et al. 2014; Duarte et al. 2013; Banerjee et al. 2016; Deidda et al. 2015a, b). Thus, we studied whether altered expression of NKCC1 and KCC2 in Syngap1+/− correlates with the observed functional differences regarding GABA-mediated actions. A significant difference in NKCC1 expression between Wild Type (WT) and Syngap1+/− at P8 was detectable using protein biochemical analysis (Fig. 2A, B). Notably, a compensatory increase in KCC2 expression was only noticeable at P8 and not at P14 in Syngap1+/− (Fig. 2C, D). At P14 Syngap1+/−, the KCC2 expression level was significantly low compared to WT mice—a similar result as our immunofluorescence analyses (Fig. 2E, F).
Age-dependent expression levels of NKCC1 and KCC2 Cl− co-transporters in WT and Syngap1+/− mice. A Top, Representative NKCC1 immunoblots at P8 are depicted. B Bottom, line graph summarizes NKCC1 expression levels at different post-natal ages for WT as well as Syngap1+/−. P4–5 (WT) N = 7, 0.48 ± 0.1, (Syngap1+/−) N = 11, 0.58 ± 0.03 (p: 0.1320); P7 (WT) N = 7, 1 ± 0.1, (Syngap1+/−) N = 8, 0.87 ± 0.1228 (p > 0.05); P8 (WT) N = 6, 0.72 ± 0.05, (Syngap1+/−) N = 6, 0.98 ± 0.09 (*p < 0.05); P14–15 (WT) N = 13, 0.8 ± 0.05, (Syngap1+/−) N = 10, 0.8 ± 0.07 (p > 0.05); P21–23 (WT) N = 7, 0.7 ± 0.03, (Syngap1+/−) N = 7, 0.68 ± 0.06 (p > 0.05); ≥ P90 (WT) N = 10, 0.7 ± 0.07, (Syngap1+/−) N = 10, 0.79 ± 0.08 (p > 0.05). F(5, 90) = 1.48, p = 0.2027 for the interaction. C Top, Representative P14–15 immunoblots for KCC2 are shown. D Bottom, line graph summarizes KCC2 expression level during development in WT as well as Syngap1+/−. (P4–5 (WT) N = 9, 2.3 ± 0.18, (Syngap1+/−) N = 9, 2.8 ± 0.18 (p > 0.05); P7 (WT) N = 8, 2.4 ± 0.17, (Syngap1+/) N = 8, 2 ± 0.17 (p > 0.05); P8 (WT) N = 5, 3.7 ± 0.12, (Syngap1+/−) N = 6, 6.2 ± 0.79 (**p < 0.01); P14-15 (WT) N = 7, 5.717 ± 0.7434, (Syngap1+/−) N = 8, 2 ± 0.3 (***p < 0.001); P21–23 (WT) N = 7, 4.872 ± 0.6442, (Syngap1+/−) N = 7, 3.4 ± 0.1 (p > 0.05); ≥ P90 (WT) N = 10, 2.3 ± 0.2, (Syngap1+/−) N = 10, 2.8 ± 0.2 (p > 0.05). F(5, 82) = 14.46, p < 0.0001 for the interaction. Data are presented as mean ± SEM. N number of mice. Two-way ANOVA Tukey's multiple comparisons test. E Representative immunofluorescence images depicting SYNGAP1 and KCC2 localisation and expression pattern in hippocampus region (DGGC for all age group and sections) in WT and Syngap1+/− mice at P8, 14–15 and ≥ 90. White arrows indicate punctate expression pattern of KCC2 and SYNGAP1. Blue colour represents the Hoescht staining of the nucleus, green coloured puncta are of SYNGAP1 protein, and KCC2 puncta surrounding soma and dendrites are presented in red. F Bar graph representing the integrated density of WT and Syngap1+/− in the individual age group is shown in front of the respective panel of P8, 14–15 and ≥ 90. P8 (WT) n = 4, 1,165,283 ± 64,324, (Syngap1+/−) n = 5, 1,426,211 ± 47,106 (*p < 0.05); P14–15 (WT) n = 7, 1,202,925 ± 43,917, (Syngap1+/−) n = 6, 766,779 ± 18,128 (***p < 0.001); P ≥ 90 (WT) n = 6, 1,697,380 ± 22,155, (Syngap1+/−) n = 8, 1,656,486 ± 32,631 (p > 0.05). Scale bar = 20 μm. Data are presented as mean ± SEM. Unpaired t-test, n: number of sections
These results point towards an irregular expression of NKCC1 and KCC2 in Syngap1+/− mice during their early developmental stages. An altered expression could potentially interrupt the ECl−and affect synaptic maturation and function. To further address a potential modulation on Nkcc1 and Kcc2 gene expression levels, qPCR analyses were performed, and no change in the transcript levels was observed within the hippocampus (Supplementary Fig. 2). Thus, our findings suggest a disruption in the post-translational processing of NKCC1 and KCC2.
6BIO induces a hyperpolarising shift in E GABA and restores LTP in Syngap1 +/− mutation
The results thus far demonstrated that a mutation at Syngap1 caused a reduced neuronal network activity and inhibition due to disabling the switch of GABA polarity during the development of transgenic mice. We sought whether administration of 6BIO might restore synaptic plasticity, EGABA, and behavioural dysfunction in Syngap1+/− mice. We chose three different age groups: Group I: P10–16 (critical period; 6BIO injected from P10–P16 daily, and behavioural testing was done at P80), Group II: P10–80 (critical period till adulthood; 6BIO injected from P10–P80 daily, and behavioural testing was done at P16, P42 for OFT, and P80 for NOR, SI, SP), and Group III: P30–80 (adulthood; 6BIO injected from P30–P80 daily, and behavioural testing was done at P80 for OFT, NOR, SI, SP; Fig. 3A). A study had demonstrated that the Syngap1 expression peaks at P14–16 where deficits in synaptic function and dendritic spine morphology was reported in Syngap1+/− mice. Further, they have demonstrated that inducing heterozygous knockout of Syngap1 in adults did not impact synaptic function contrary to knockout of Syngap1 in P0 (Clement et al. 2012). Correspondingly, our data shows that KCC2 expression and function (EGABA) is mainly disrupted at P14–16. Thus, we chose this period as a critical period of development as most of our study involved the hippocampus (critical period of development ends at 3-week) (Chakraborty et al. 2021).
6BIO hyperpolarises GABA reversal potential at P15–16 in Syngap1+/− mice and corrects LTP in the post-critical period of development. A Chart depicting the regime for 6BIO intraperitoneal injection (5 mg/kg) and categorisation of age groups. B Grouped data for EGABA estimated from I–V curve from individual WT (Vehicle and 6BIO treated) and Syngap1+/− mice (Vehicle and 6BIO treated). WT-Vehicle: N = 5, n = 5; Syngap1+/−-Vehicle: N = 4, n = 6; WT-6BIO: N = 3, n = 5; Syngap1+/−-6BIO: N = 3, n = 7; F(1,19) = 9.761, p = 0.0056 C For Group II, where injections were done from P10–80, WT-Vehicle: N = 4, n = 8; Syngap1+/−-Vehicle: N = 4, n = 7; WT-6BIO: N = 4, n = 8; Syngap1+/−-6BIO: N = 4, n = 6, F(1,25) = 2.047, p = 0.1649 and D Group III, where injections were done from P30–80, WT-Vehicle: N = 3, n = 6; Syngap1+/−-Vehicle: N = 4, n = 6; WT-6BIO: N = 5, n = 9; Syngap1+/−-6BIO: N = 5, n = 9, F(1,26) = 4.731, p = 0.0389, slope of fEPSP was normalised to mean value of 15-min baseline period and 45-min post-LTP recordings were performed. Example traces are average of those recorded in 1–2 min around the time point indicted (I and II). Two-way ANOVA, Tukey’s multiple comparisons test
Considering the impact of Syngap1+/− mutation as reported in other brain regions (Clement et al. 2012, 2013; Aceti et al. 2015; Ozkan et al. 2014), we performed EGABA from DGGC and LTP from the Schaffer-collateral commissural pathway to validate the effect of 6-BIO in different regions of the hippocampus. 6BIO was intraperitoneally injected to mice from P10 to P16 daily, and ECl− recordings were obtained at either P15 or P16 after an hour of injection. We noticed that ECl− was corrected in 6BIO treated Syngap1+/− mice at P10–16 (Fig. 3B); thus, it could rectify increased excitation. Similarly, we observed that LTP was restored in 6BIO treated Syngap1+/− mice, particularly after the critical period of development (LTP at P80; P10–80: Fig. 3C; LTP at P80; P30–80: Fig. 3D). Injection paradigm for Group II: P10–80; 6BIO injection was from P10–P80 daily, and field recordings were done at P80; and Group III: P30–80; 6BIO injection was from P30–P80 daily, and field recordings were done at P80. Further, for the Input/Output (I/O) curve, linear regression was performed for each slice and slope of the relationship was determined, followed by one-way ANOVA on regression points, for establishing statistical difference for relationship. We had not observed a change in the I/O curve between the genotypes (WT versus Syngap1+/− mice; vehicle as well as 6BIO treated) in both the age groups, Group II and Group III (Supplementary Fig. 3A, D), which corroborates with the previous publications (Clement et al. 2012; Ozkan et al. 2014). Our results suggest 6BIO interventions, particularly after the critical period of development, can correct functional deficits in Syngap1+/− mice.
6BIO prevents behavioural deficits of Syngap1 +/− mice
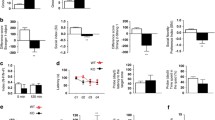
Open field test (OFT), at P80; P10–16, 6BIO treated Syngap1+/− mice showed partial correction of hyperactivity (Fig. 4A). Interestingly, correction of hyperactivity was observed in mice at P16; P10–80; Fig. 4B. The effect of 6BIO became less pronounced at P42; P10–80; Fig. 4C, and no rescue was observed at P80; P30–80; Fig. 4G. The data suggest that 6BIO is most efficient when administered during the critical period of development. In addition, our experiments demonstrated that anxiety was corrected when behaviour was performed at P80; P10–16, Fig. 4D; P16; P10–80, Fig. 4E; P42; P10–80, Fig. 4F; and a partial correction for P30–80 (Fig. 4H) age group. Partial correction indicates a significant difference between WT-Vehicle and Syngap1+/−-6BIO, but Syngap1+/−-6BIO was significantly different than Syngap1+/−-vehicle (not reached WT level but performing better than Syngap1+/−-vehicle). Thus, in contrast to the previous conclusion, the data suggest that anxiety is more efficiently corrected when 6BIO is administered after the critical period of development.
6BIO corrects hyperactivity, anxiety and seizure threshold in Syngap1+/−. Total distance travelled by the mice in open field chamber was grouped and shown as individual data points for P10-16 age group where behaviour was performed at P80, total distance travelled A WT-Vehicle: N = 11; Syngap1+/−-Vehicle: N = 9; WT-6BIO: N = 12; Syngap1+/−-6BIO: N = 13, F(1, 41) = 1.676, p = 0.2027, and number of entries. B P10–80 where behaviour was done at P16, WT-Vehicle: N = 20; Syngap1+/−-Vehicle: N = 11; WT-6BIO: N = 16; Syngap1+/−-6BIO: N = 14, F(1, 57) = 14.83, p = 0.0003, C P10–80 where behaviour was done at P42, WT-Vehicle: N = 17; Syngap1+/−-Vehicle: N = 9; WT-6BIO: N = 15; Syngap1+/−-6BIO: N = 9, F(1, 44) = 4.752, p = 0.0347, and G P30–80, WT-Vehicle: N = 14; Syngap1+/−-Vehicle: N = 14; WT-6BIO: N = 16; Syngap1+/−-6BIO: N = 16, F(1, 56) = 0.4686, p = 0.4965. D Number of entries in the centre as a measure of anxiety was grouped and shown as individual data points for P10–16 age group where behaviour was performed at P80, WT-Vehicle: N = 12; Syngap1+/−-Vehicle: N = 11; WT-6BIO: N = 12; Syngap1+/−-6BIO: N = 14, F(1, 45) = 0.8829, P = 0.3524. E P10–80 where behaviour was done at P16, WT-Vehicle: N = 20; Syngap1+/−-Vehicle: N = 11; WT-6BIO: N = 16; Syngap1+/−-6BIO: N = 14, F(1, 57) = 18.01, p < 0.0001, F P10–80 where behaviour was done at P42, WT-Vehicle: N = 18; Syngap1+/−-Vehicle: N = 10; WT-6BIO: N = 15; Syngap1+/−-6BIO: N = 11, F(1, 50) = 2.194, p = 0.1449, and H P30–80, WT-Vehicle: N = 13; Syngap1+/−-Vehicle: N = 12; WT-6BIO: N = 15; Syngap1+/−-6BIO: N = 18, F(1, 54) = 6.002, p = 0.0176. I Mice were injected 6BIO from P30–80; thereafter, fluoroethyl-based seizure threshold was evaluated and plotted as grouped data showing individual points for tonic–clonic seizure. 6BIO increased the threshold for tonic–clonic, similar to WT levels, in Syngap1+/−. F(1,25) = 3.354, p = 0.0790, WT-Vehicle: N = 9; Syngap1+/−-Vehicle: N = 7; WT-6BIO: N = 7; Syngap1+/−-6BIO: N = 6. Two-way ANOVA Tukey’s multiple comparisons test
A primary attribute of ID/ASD is epileptic seizures observed in patients with SYNGAP1 haploinsufficiency (Hamdan et al. 2009) and reduced threshold to fluoroethyl in patients Syngap1+/− mice (Clement et al. 2012). We observed a normalised seizure threshold for the tonic–clonic seizure that matched WT levels in 6BIO administered Syngap1+/− mice at P80 for the P30–80 age group (Fig. 4I). We evaluated the duration for the same and found a trend in increased duration of the tonic–clonic seizure (equivalent to WT levels) in Syngap1+/− mice administered with 6BIO, which was statistically non-significant to the Syngap1+/−-vehicle (p = 0.1636) (Supplementary Fig. 4A). The intensity was non-significant among groups suggesting that 6BIO did not affect the seizure severity in Syngap1+/− mice (Supplementary Fig. 4B).
To investigate the effect of 6BIO on memory, we performed a novel object recognition test (NOR; Familiar vs novel object). All behaviour tasks were performed at P80 (P10–16: Fig. 5A; P10–80 Fig. 5B; P30–80 Fig. 5C). Remarkably, we found that 6BIO substantially restored memory performance in Syngap1+/− mice.
6BIO corrects memory recognition and sociability deficits in Syngap1+/− particularly in the post-critical period of development: A Discrimination index was plotted as the measure of recognition memory and plotted as individual data points for P10–16 where behavior was done at P80, WT-Vehicle: N = 11; Syngap1+/−-Vehicle: N = 6; WT-6BIO: N = 9; Syngap1+/−-6BIO: N = 14, F(1, 36) = 14.76, p = 0.0005, B P10–80 where behavior was done at P80, WT-Vehicle: N = 16; Syngap1+/−-Vehicle: N = 7; WT-6BIO: N = 11;: N = 10, F(1, 40) = 1.982, p = 0.1669, and C P30-80 where behavior was done at P80, WT-Vehicle: N = 8; Syngap1+/−-Vehicle: N = 7; WT-6BIO: N = 9; Syngap1+/−-6BIO: N = 15, F(1, 35) = 7.687, p = 0.0089. Social interaction and preference was evaluated as time spent with stranger1 as compared to empty jar, and time spent with stanger2 as compared to stranger1 respectively for D P10-16 where behavior was done at P80, WT-Vehicle: N = 12; Syngap1+/−-Vehicle: N = 10; WT-6BIO: N = 12; Syngap1+/−-6BIO: N = 14, F(3, 88) = 1.481, p = 0.2252, G WT-Vehicle: N = 12; Syngap1+/−-Vehicle: N = 10; WT-6BIO: N = 12; Syngap1+/−-6BIO: N = 14, F(3, 88) = 0.6549, p = 0.5820, E P10–80 where behavior was done at P80, WT-Vehicle: N = 9; Syngap1+/−-Vehicle: N = 6; WT-6BIO: N = 10; Syngap1+/−-6BIO: N = 10, F(3, 62) = 3.722, p = 0.0158, H WT-Vehicle: N = 9; Syngap1+/−-Vehicle: N = 8; WT-6BIO: N = 10; Syngap1+/−-6BIO: N = 10, F(3, 66) = 2.692, p = 0.0532, and F P30–80 where behavior was done at P80, N = 12; Syngap1+/−-Vehicle: N = 14; WT-6BIO: N = 12; Syngap1+/−-6BIO: N = 18, F(3, 104) = 2.909, p = 0.0381, I WT-Vehicle: N = 12; Syngap1+/−-Vehicle: N = 13; WT-6BIO: N = 12; Syngap1+/−-6BIO: N = 18, F(3, 102) = 2.616, p = 0.0551. Two-way ANOVA Tukey's multiple comparisons test
Similar to NOR, a social novelty test (Social Interaction (SI) and Social preference (SP)) was performed for all age groups at P80 (P10–16: Fig. 5D, G; P10–80: Fig. 5E, H; P30–80: Fig. 5F, I). Our results suggested that the time spent with stranger-1 compared to the empty jar for 6BIO treated Syngap1+/− mice were comparable to WT levels in all age groups. However, we did not observe rescue in social preference for 6BIO treated Syngap1+/− mice in the P10–16 group. Overall, our results suggest that 6BIO effectively corrected the SI and SP deficits when administered after a critical period of development in Syngap1+/− mice. We have summarised all the behaviour results in Table 3. The results indicate that rescuing GABAergic-synaptic function is fundamental in correcting behaviour in Syngap1+/− mice.
Discussion
The interplay between GABAergic and Glutamatergic systems is indispensable for the formation, maturation, refinement, and maintenance of synapses in the developing brain. Aberration in any of these processes results in neurodevelopmental and psychiatric disorders due to persistent changes in neuronal activity during development (Ben-Ari et al. 1989; Cherubini et al. 1991; Owens et al. 1996; Rivera et al. 1999). Based on our results, we consider abnormal activation of neurons to occur upon GABA activation during developmental stages due to increased NKCC1 levels at P8 and a concomitant increase in KCC2 levels at P8 in Syngap1+/−, contributing to the increased intrinsic excitability (Clement et al. 2012; Kaila et al. 2014), similar to Fragile-X and Rett syndrome (Hinz et al. 2019; Banerjee et al. 2016). Increased KCC2 expression levels at P8 may further contribute to the intrinsic excitability, as demonstrated by an intriguing interaction with the Na+–K+-ATPase pump (Ikeda et al. 2004). Further, disrupted KCC2 expression may compensate for the increase in AMPA/NMDA ratio at P14–15, causing increased excitability of the neurons at the developmental stage (2-week; Supplementary Fig. 4), which is linked with synaptogenesis through KCC2 interactions with cytoskeletons such as 4.1, and ANKYRIN to regulate AMPAR clustering at the postsynapse (Chevy et al. 2015; Li et al. 2007; Llano et al. 2015; Chamma et al. 2012; Clement et al. 2012; Ikeda et al. 2004). Our results obtained by KCC2 expression at P14–15 corroborated with EGABA results (discussed later), and not NKCC1 expression, prompted us to verify the KCC2 protein expression results by immunohistochemical analysis complemented immunoblotting results.
We propose that the irregular EGABA and subsequent depolarisation could lead to the disinhibition of GABA. An increase in the mIPSC amplitude at P14–15 (Clement et al. 2012) indicating elevated GABA receptors at the postsynapses, thus, compensating for the reduced expression of KCC2 at P14–15. Enhanced excitation through disinhibition is reflected by disrupted network activity mediated by GABA in P8–9 and P14–15, further corroborated by less tonic inhibition in 2-week-old Syngap1+/− mice. Less network activity in P14–15 indicates abnormality either in the compensatory effect of increased excitation due to disrupted GABA polarity switch or the decreased expression of KCC2 at the postsynapses in Syngap1+/−.
It is shown that the coordinated action of GABAergic and glutamatergic synaptic inputs result in GABA-mediated spontaneous giant inward currents (GICs) in the early postnatal stages of development. GICs are network-driven synaptic potentials that are synchronous and characterised by recurrent membrane depolarisation (Ben-Ari et al. 1989). Besides, neuronal activity, crucial for GICs, is facilitated by membrane depolarisation by GABAA receptors (synaptic and extrasynaptic), which is strengthened by the activation of persistent Sodium currents (Sipila et al. 2006). Therefore, the possibility of involvement of glutamatergic activity and inward Sodium currents on the generation of GICs cannot be ignored and considered during recordings.
Given that the GIC is an effect of the activity of the population of neurons, and we wanted to measure GIC under physiological conditions, we performed the recordings in the voltage-clamp mode at − 70 mV. In various studies, steady-state and transient inactivation of Sodium channels were determined with Boltzmann functions and half maximum voltage was found to be − 67 to − 65 mV, which implied activation at more depolarised potentials, and Sodium channels would be inactivated at − 70 mV in our recordings (Magee and Johnston 1995; Park et al. 2013; Migliore et al. 1999; Carter et al. 2012). Thus, it excludes the possibility of participation of inward Sodium currents at − 70 mV in our GIC recordings. Further, we analysed the width of the action potential, which does not overlap with the width of the initial rising part of GIC, indicating that the inward sodium currents are not contributing to the GICs (Supplementary Fig. 1C, D). Moreover, in the single GIC example trace with expanded timescale shown in the supplementary Fig. 1D, we did not observe spikes on top of GIC, suggesting a lack of involvement of inward sodium currents.
Further, our main objective in this study was to identify whether GABA contributes to the excitatory activity of neurons at P9 and P14 in the Syngap1+/− mice. Our data indicate that, in the presence of Bicuculline, there was a reduction of the number of GICs in Syngap1+/− mice, suggestive of GABA being still excitatory in these mice. Given that the GIC was not entirely abolished in the presence of Bicuculline in Syngap1+/− mice, the responses measured also indicates the role of glutamatergic activity in generating GIC at P9 and P14.
Our study, so far, has shown reduced GABA activity at P14–16 suggesting a possibility of less network activity (expanded data 1D) and decreased GABA-mediated activity in the presence of Bicuculline. This result indicates that GABA is still excitatory, which may fail to compensate for increased AMPA/NMDA ratio and mEPSC/mIPSC amplitude observed in Syngap1+/− mice at P14–15 (Clement et al. 2012; Ozkan et al. 2014). The depolarisation observed in 2-week-old Syngap1+/− mice could be due to the disinhibition of GABA during early stages of development that results from impaired compensation of GABA, thereby leading to altered E/I as observed in Syngap1+/− mice (Ozkan et al. 2014; Clement et al. 2012). Thus, we hypothesise that, based on our study and other published results, the cumulative impact of increased AMPA/NMDA, mEPSC, mIPSC, positive EGABA, and decreased giant depolarisation potentials, potentially indicating disruption to the neuronal circuit activity at P14–16 leading to E/I imbalance, altered critical period of plasticity, impaired physiological, behavioural, and cognitive functions in the Syngap1+/− mice.
Previous studies have demonstrated that the synaptic function and dendritic spine morphology deficits in Syngap1+/− mice were observed either over a single day or in a 2–3-day period within the critical period of development which is comparable with our observation: hippocampus—critical period ends at 3-week; somatosensory—7–9-day; mPFC—7–8-day; layer 5–30-day (Clement et al. 2012, 2013; Ozkan et al. 2014; Aceti et al. 2015). These earlier studies strongly imply the stringent regulation of synaptic function and spine morphology during the critical period of development by SYNGAP1 and how a mutation in this gene can disrupt it. Besides, similar to Syngap1+/−mice, disruption in the synaptic function and spine morphology in a comparable window of the critical period of development was reported in the Fragile-X syndrome mouse model (Harlow et al. 2010). Thus, these studies and our study indicate that the changes observed due to mutation in genes implicated in neurodevelopmental disorders regulate the critical period of development in a concise and accurate window of time.
Gating the critical period of plasticity depends on optimal inhibition during the development, which determines the fate of inhibitory circuits in the brain (Hensch 2005; Hubener and Bonhoeffer 2014; Duarte et al. 2013; Verma et al. 2019; He et al. 2014). Selective pharmacological modulations in GABAergic circuits during development have shown promising results in rescuing several physiological and behavioural deficits in ID/ASD models (Banerjee et al. 2016; Deidda, et al. 2015a, b; He et al. 2019; Braat and Kooy 2015), thus, a key candidate target for therapeutic intervention. Restoring the protein level of SYNGAP1 in adult stages restored synaptic function (LTP) but not behaviour, suggesting that these neurons are hard-wired during development (Ozkan et al. 2014). Nevertheless, genetic restoration of Syngap1 in the adult stages has restored some of the phenotypes, implying that not all deficits related to Syngap1+/− can be ascribed to neuronal circuit damage caused by aberrant neurodevelopment (Creson et al. 2019). A significant caveat evaluating the efficacy of drugs in pre-clinical models is either the lack of investigation on the effectiveness of drugs after the critical period (during hard-wiring) or the inability of the compound to restore function after the critical period (after hard-wiring) (He et al. 2019; McCamphill et al. 2020). Thus, concise therapeutics to correct deficiencies observed in Syngap1+/−-mediated ID/ASD is unfounded and provides the platform to find a candidate target for therapeutics that can restore phenotypes after the neurodevelopment period.
In various studies, targeting the GABAergic circuit has improved synaptic and behavioural deficits observed in the murine models of neurodevelopmental disorders (Banerjee et al. 2016; Deidda, et al. 2015a, b; He et al. 2019; Braat and Kooy 2015; Cellot and Cherubini 2014; Savardi et al. 2020; D’Hulst and Kooy 2007; Raveendran et al. 2020). Thus, we speculated that restoring GABAergic function to the WT levels can correct behavioural deficits in Syngap1+/− mice. Our initial findings described the altered function of Chloride co-transporters (reversal potential of GABA) at P14–15 was restored when 6BIO was administered in Syngap1+/− mice. In support of this, a recent study has shown that administering the BIO compound in a Rett syndrome model restored EGABA, thus, restoring behavioural function (Tang et al. 2019). This study also led us to hypothesise that rectifying GABA function can correct behavioural deficits in Syngap1+/− mice.
It is essential to understand whether the drug can correct behavioural deficits when administered in young adolescent stages, apart from when given during the critical period of development (P10–16). Based on the paradigm in Fig. 3A, P10–16 will address whether targeting the critical period alone would be sufficient to correct the phenotype with lasting effects in adult stages. P10–80 will answer whether the drug should be administered from the critical period of development and continue throughout adulthood. P30–80, the most crucial aspect of the drug discovery, would imply whether administration of the drug after a critical period of development corrects synaptic dysfunction and behavioural deficits, which remains a challenge to date. Most compounds can restore these functions when administered during the critical period of development (i.e., P10–16), which makes our study unique and different from the other published results.
We have recently shown that 6-bromoindirubin-3ʹ-oxime (6BIO) is neuroprotective in an MPTP-based mouse model of Parkinson’s disease, suggesting the potency to cross the blood–brain barrier and inhibit GSK-3β in neurons (Suresh et al. 2017). 6BIO administration in P30–80 (group III) has substantially restored functional (LTP, EGABA (P16)) and behavioural deficits (sociability, memory, and anxiety) in Syngap1+/− mice. However, hyperactivity was not corrected after the neurodevelopmental period suggesting that the target period is during the critical period of development. It appears that social isolation, social preference, and seizure threshold were not impacted by the hard wiring of the neuronal circuit during development as it is corrected in all three strategies, except in group I for SP. Primarily, we demonstrate that administration of 6BIO corrected memory deficits in all therapeutic strategies, particularly after the neurodevelopmental period.
Though we have observed the LTP (Fig. 3C), hyperactivity (Fig. 4B), and anxiety (Fig. 4E) can be correlated to the normalisation of reversal potential of chloride at P15 and P16; P10–16, in the Syngap1+/− mice. However, we believe that inhibition of GSK-3β after P30 (P30–80 group) may directly play a role in regulating the GABAergic neuronal function as demonstrated in the mouse model of Grin1 hypofunction, using a paralog-selective GSK-3β inhibitor (BRD3731). BRD3731 restored evoked-gamma oscillations, baseline gamma power, spatial working memory, and paired-pulse inhibition of acoustic startle in the NMDAR hypofunction mouse model when intraperitoneally injected in adult mice (Nakao et al. 2020). An essential additive of gamma-oscillations is GABAA receptor-mediated inhibition (Buzsaki and Wang 2012), which is dampened in Syngap1+/− mice (Sullivan et al. 2020; Berryer et al. 2016). Gamma-oscillations are observed in different brain regions such as the neocortex, entorhinal cortex, amygdala, hippocampus, striatum and thalamus (Buzsaki and Wang 2012) and provide a fundamental platform for cognition (Fries 2009). Therefore, given that the SYNGAP1 is downstream of NMDAR and presented with memory and behavioural deficits, like in Grin1 mutant mice, we consider 6BIO to regulate GABAergic neuronal functions in adult stages, as observed in our data. Gamma-oscillations are observed in different regions of the brain, including those overlapping with the regions regulating memory (hippocampus), SP (amygdala), NOR (hippocampus and entorhinal cortex), which was shown to be altered in Syngap1+/− mice. GABAA-receptor-mediated inhibition subserves as a pivot among all these events. Hence, we hypothesise that 6BIO may act on the GABA-mediated functions such as gamma-oscillations, which would have corrected the excitation-inhibition imbalance, cognitive deficits, and behavioural deficits in adult stages. However, these will be investigated in future experiments.
Absence of rescue of SP at P10–16—behaviour performed at P80—compared to P10–80 and P30–80 groups may not be directly influenced by neonatal E/I imbalance, contrary to NOR and SI at P10–16. One possible mechanism could be that the regions involved in Social Preference (SP), particularly the connections from the amygdala to the mPFC, may not mature. The critical period of development for these connections would be after P10–16, as reflected in the restoration of SP in our P10–80 group. These observations corroborate the earlier findings made in these studies (Tottenham and Gabard-Durnam 2017; Huang et al. 2020). They have postulated that the sensory periods for the amygdala and mPFC is stronger not during infancy but after childhood, i.e. after ~ P20 in mice. In addition, Huang et al. have shown that optogenetic modulation of the mPFC-Amygdala disrupted the social preference in mice (Huang et al. 2020; Ferri et al. 2016). Thus, the maturation of mPFC-Amygdala (or vice versa) during the critical period of development is crucial to distinguish two different mice in SP. Therefore, when we injected 6BIO from P10–16, we would have missed the critical period of development. The compound may not adequately affect the neurons and their network to restore the functions as shown in our results.
Further, studies have shown that the region involved in NOR are the hippocampus, perirhinal, entorhinal, and parahippocampal cortex. While the perirhinal cortex is involved in object recognition after a short interval, the hippocampus is required for long term object recognition (Antunes and Biala 2012; Reger et al. 2009). Based on lesion studies in the hippocampus and the perirhinal cortex, it is evident that the hippocampus is involved in object memorisation by encoding the experience of the object. In contrast, the perirhinal cortex is involved in object recognition once it is necessary to represent basic information about the familiarity or novelty of an object. Considering the significant role of the hippocampus in object discrimination, and Syngap1 heterozygous mutation has a drastic impact on the synaptic function and structure at P14–16, 6BIO administration at P10–16, P10–80 restored the NOR in these mice. Further, these studies and our early discussion on the effect of 6BIO on gamma oscillation would explain our observation where NOR, and SI and SP deficits were restored in the adult stages, which are independent of neonatal E/I imbalance. Based on this account of the function of the hippocampus in object discrimination and recognition and our earlier discussion that the late critical period of development of Amygdala-mPFC connections to distinguish between two mice, we hypothesise that, during SI test, the 6BIO administration at P10–16 and P10–80 would have facilitated to distinguish an object from the mouse. Thus, it would have enabled the Syngap1 mutant mice to spend more time with the novel mouse than the object, which requires further investigation using optogenetics and a top-down approach.
Hyperexcitable networks resulting in seizures during early development may underlay increased seizure susceptibility and behavioural deficits in adulthood in Syngap1+/− mice. It has been shown that neonatal induced seizures result in deficits in spatial learning and memory (demonstrated as an increased time of training of mice in Morris water maze), and deficits in social behaviour of the mice (seizure mice spent more time with the object rather than mice and equal time with familiar and unfamiliar mice) (Lugo et al. 2014; Avoli 2012). involved in seizures/epilepsy are the hippocampus, amygdala, thalamus, cerebellum and prefrontal cortex Bromfield et al. (2006). These regions overlap with the regions involved in regulating social behaviour and memory. Syngap1+/− mice are prone to neonatal seizures, and it could be one of the potential causes of the phenotypes observed in the adult. This observation could also explain the restoration of memory and social behaviour at P80 on the daily administration of 6BIO from P30–P80. It suggests an alternative mechanism where 6BIO may regulate GABAergic neurotransmission (in epilepsy/seizure, GABAergic neurotransmission is less), thus, restoring phenotypes as observed in the adult Syngap1+/− mice.
Studies have demonstrated that the deficits observed in the Dentate gyrus (DG) in Syngap1+/− is recapitulated in the CA1 region of the hippocampus (Clement et al. 2012). It is shown that LTP was reduced in the CA3–CA1 region while the rest of the study was from the forebrain region of the brain (Ozkan et al. 2014). As demonstrated by previous publications, the effect observed in DG is recapitulated in other parts of the hippocampus and other parts of the brain, such as the Barrel cortex and mPFC (Clement et al. 2013). Thus, these data demonstrate that the impact of Syngap1+/− global and that prompted us to investigate the effect of 6BIO in the DGGC and CA1 region of the hippocampus. We believe that rectifying the EGABA may correct synaptic function and plasticity as optimal GABA function is needed for suitable excitatory synaptic function leading to plasticity (Ormond and Woodin 2011; Larson and Munkacsy 2015).
The reason we tested 6BIO is that studies from Franklin et al 2014, Hooper et al, 2007, and McCampil et al. 2020 have demonstrated that inhibiting GSK3ß attenuated LTP deficits observed in Fragile-X models (Franklin et al. 2014; Hooper et al. 2007; McCamphill et al. 2020), similar to what we observed in Syngap1+/− model. However, these studies had not shown the level of rescue we observed in Syngap1+/− mice when 6BIO was administered after the critical period of development (i.e., adolescent young adults). Despite this, there is a lack of clarity on the function of GSK-3ß in modulating synaptic function. The reason, we believe, is that the pharmacological approaches and shRNA experiments (Liu et al. 2017) could be more acute compared to the conditional deletion, while the latter may induce the risk of measuring secondary effects of GSK-3β deletion. We agree that 6BIO may have other targets that can restore the synaptic function and behaviour deficits to WT levels which need to be explored but not in the scope of this study.
In conclusion, our results are extremely encouraging as the administration of 6BIO after the neurodevelopmental period amended restored most of the phenotypes evaluated and the synaptic functions. These results further emphasise the regime for therapeutic intervention should target the developmental period to protect the brain from the potential hard-wired circuit damage and maximise the correction of phenotype. Our results denote a general mechanism involving GABAergic signalling prevailing in several neurodevelopmental disorders that 6BIO can correct. However, a significant limitation of this compound is that it is not water-soluble, and derivates of the water-soluble compound should be synthesised to administer it orally. Thus, our study supports the possibility that cognitive, emotional, and social symptoms that result from hard-wired neuronal circuit damage during development may still be corrected by late pharmacological intervention in adulthood.
Availability of data and materials
All raw data obtained for each experiment is available on request.
Code availability
Not applicable.
References
Aceti M, Creson TK, Vaissiere T, Rojas C, Huang WC, Wang YX, Petralia RS, Page DT, Miller CA, Rumbaugh G (2015) Syngap1 haploinsufficiency damages a postnatal critical period of pyramidal cell structural maturation linked to cortical circuit assembly. Biol Psychiatry 77:805–815
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Avoli M (2012) Herbert H. Jasper and the basic mechanisms of the epilepsies. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV (eds) Jasper’s basic mechanisms of the epilepsies, 4th edn. National Center for Biotechnology Information (US), Bethesda, MD
Banerjee A, Rikhye RV, Breton-Provencher V, Tang X, Li C, Li K, Runyan CA, Fu Z, Jaenisch R, Sur M (2016) Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc Natl Acad Sci USA 113:E7287–E7296
Bear MF, Huber KM, Warren ST (2004) The mGluR theory of fragile X mental retardation. Trends Neurosci 27:370–377
Ben-Ari Y (2002) Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci 3:728–739
Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL (1989) Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol 416:303–325
Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E (2012) The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 18:467–486
Berryer MH, Hamdan FF, Klitten LL, Moller RS, Carmant L, Schwartzentruber J, Patry L, Dobrzeniecka S, Rochefort D, Neugnot-Cerioli M, Lacaille JC, Niu Z, Eng CM, Yang Y, Palardy S, Belhumeur C, Rouleau GA, Tommerup N, Immken L, Beauchamp MH, Patel GS, Majewski J, Tarnopolsky MA, Scheffzek K, Hjalgrim H, Michaud JL, Di Cristo G (2013) Mutations in SYNGAP1 cause intellectual disability, autism, and a specific form of epilepsy by inducing haploinsufficiency. Hum Mutat 34:385–394
Berryer MH, Chattopadhyaya B, Xing P, Riebe I, Bosoi C, Sanon N, Antoine-Bertrand J, Levesque M, Avoli M, Hamdan FF, Carmant L, Lamarche-Vane N, Lacaille JC, Michaud JL, Di Cristo G (2016) Decrease of SYNGAP1 in GABAergic cells impairs inhibitory synapse connectivity, synaptic inhibition and cognitive function. Nat Commun 7:13340
Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, Friauf E, Nothwang HG (2006) Oligomerisation of KCC2 correlates with development of inhibitory neurotransmission. J Neurosci 26:10407–10419
Blaesse P, Airaksinen MS, Rivera C, Kaila K (2009) Cation-chloride cotransporters and neuronal function. Neuron 61:820–838
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Booth CA, Brown JT, Randall AD (2014) Neurophysiological modification of CA1 pyramidal neurons in a transgenic mouse expressing a truncated form of disrupted-in-schizophrenia 1. Eur J Neurosci 39:1074–1090
Braat S, Kooy RF (2015) The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron 86:1119–1130
Bromfield EB, Cavazos JE, Sirven JI (eds) (2006) An introduction to epilepsy. American Epilepsy Society, West Hartford (CT)
Buzsaki G, Wang XJ (2012) Mechanisms of gamma oscillations. Annu Rev Neurosci 35:203–225
Carter BC, Giessel AJ, Sabatini BL, Bean BP (2012) Transient sodium current at subthreshold voltages: activation by EPSP waveforms. Neuron 75:1081–1093
Cellot G, Cherubini E (2014) GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr 2:70
Chakraborty R, Vijay Kumar MJ, Clement JP (2021) Critical aspects of neurodevelopment. Neurobiol Learn Mem 180:107415
Chamma I, Chevy Q, Poncer JC, Levi S (2012) Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front Cell Neurosci 6:5
Cherubini E, Gaiarsa JL, Ben-Ari Y (1991) GABA: an excitatory transmitter in early postnatal life. Trends Neurosci 14:515–519
Chevy Q, Heubl M, Goutierre M, Backer S, Moutkine I, Eugene E, Bloch-Gallego E, Levi S, Poncer JC (2015) KCC2 gates activity-driven ampa receptor traffic through cofilin phosphorylation. J Neurosci 35:15772–15786
Clement JP, Aceti M, Creson TK, Ozkan ED, Shi Y, Reish NJ, Almonte AG, Miller BH, Wiltgen BJ, Miller CA, Xu X, Rumbaugh G (2012) Pathogenic SYNGAP1 mutations impair cognitive development by disrupting maturation of dendritic spine synapses. Cell 151:709–723
Clement JP, Ozkan ED, Aceti M, Miller CA, Rumbaugh G (2013) SYNGAP1 links the maturation rate of excitatory synapses to the duration of critical-period synaptic plasticity. J Neurosci 33:10447–10452
Cope DW, Di Giovanni G, Fyson SJ, Orban G, Errington AC, Lorincz ML, Gould TM, Carter DA, Crunelli V (2009) Enhanced tonic GABAA inhibition in typical absence epilepsy. Nat Med 15:1392–1398
Creson TK, Rojas C, Hwaun E, Vaissiere T, Kilinc M, Jimenez-Gomez A, Holder JL Jr, Tang J, Colgin LL, Miller CA, Rumbaugh G (2019) Re-expression of SynGAP protein in adulthood improves translatable measures of brain function and behavior. Elife 8:e46752
Deidda G, Allegra M, Cerri C, Naskar S, Bony G, Zunino G, Bozzi Y, Caleo M, Cancedda L (2015a) Early depolarising GABA controls critical-period plasticity in the rat visual cortex. Nat Neurosci 18:87–96
Deidda G, Parrini M, Naskar S, Bozarth IF, Contestabile A, Cancedda L (2015b) Reversing excitatory GABAAR signaling restores synaptic plasticity and memory in a mouse model of Down syndrome. Nat Med 21:318–326
D’Hulst C, Kooy RF (2007) The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci 30:425–431
Duarte ST, Armstrong J, Roche A, Ortez C, Perez A, O’Callaghan Mdel M, Pereira A, Sanmarti F, Ormazabal A, Artuch R, Pineda M, Garcia-Cazorla A (2013) Abnormal expression of cerebrospinal fluid cation chloride co-transporters in patients with Rett syndrome. PLoS ONE 8:e68851
Ferland RJ (2017) The Repeated Flurothyl Seizure Model in Mice. Bio Protoc 7(11):e2309. https://doi.org/10.21769/BioProtoc.2309
Ferri SL, Kreibich AS, Torre M, Piccoli CT, Dow H, Pallathra AA, Li H, Bilker WB, Gur RC, Abel T, Brodkin ES (2016) Activation of basolateral amygdala in juvenile C57BL/6J mice during social approach behavior. Neuroscience 335:184–194
Franklin AV, King MK, Palomo V, Martinez A, McMahon LL, Jope RS (2014) Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice. Biol Psychiatry 75:198–206
Fries P (2009) Neuronal gamma-band synchronisation as a fundamental process in cortical computation. Annu Rev Neurosci 32:209–224
Gamba G (2005) Molecular physiology and pathophysiology of electroneutral cation-chloride co-transporters. Physiol Rev 85:423–493
Griguoli M, Cherubini E (2017) Early correlated network activity in the hippocampus: its putative role in shaping neuronal circuits. Front Cell Neurosci 11:255
Guo X, Hamilton PJ, Reish NJ, Sweatt JD, Miller CA, Rumbaugh G (2009) Reduced expression of the NMDA receptor-interacting protein SynGAP causes behavioral abnormalities that model symptoms of Schizophrenia. Neuropsychopharmacology 34:1659–1672
Hamdan FF, Daoud H, Piton A, Gauthier J, Dobrzeniecka S, Krebs MO, Joober R, Lacaille JC, Nadeau A, Milunsky JM, Wang Z, Carmant L, Mottron L, Beauchamp MH, Rouleau GA, Michaud JL (2011) De novo SYNGAP1 mutations in nonsyndromic intellectual disability and autism. Biol Psychiatry 69:898–901
Hamdan FF, Gauthier J, Spiegelman D, Noreau A, Yang Y, Pellerin S, Dobrzeniecka S, Cote M, Perreau-Linck E, Carmant L, D’Anjou G, Fombonne E, Addington AM, Rapoport JL, Delisi LE, Krebs MO, Mouaffak F, Joober R, Mottron L, Drapeau P, Marineau C, Lafreniere RG, Lacaille JC, Rouleau GA, Michaud JL, Group Synapse to Disease (2009) Mutations in SYNGAP1 in autosomal nonsyndromic mental retardation. N Engl J Med 360:599–605
Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A (2010) Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron 65:385–398
He Q, Nomura T, Xu J, Contractor A (2014) The developmental switch in GABA polarity is delayed in fragile X mice. J Neurosci 34:446–450
He Q, Arroyo ED, Smukowski SN, Xu J, Piochon C, Savas JN, Portera-Cailliau C, Contractor A (2019) Critical period inhibition of NKCC1 rectifies synapse plasticity in the somatosensory cortex and restores adult tactile response maps in fragile X mice. Mol Psychiatry 24:1732–1747
Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6:877–888
Hinz L, Torrella Barrufet J, Heine VM (2019) KCC2 expression levels are reduced in post mortem brain tissue of Rett syndrome patients. Acta Neuropathol Commun 7:196
Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T, Hernandez F, Anderton B, Rosenblum K, Bliss T, Cooke SF, Avila J, Lucas JJ, Giese KP, Stephenson J, Lovestone S (2007) Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci 25:81–86
Huang WC, Zucca A, Levy J, Page DT (2020) Social behavior is modulated by valence-encoding mPFC-Amygdala sub-circuitry. Cell Rep 32:107899
Hubener M, Bonhoeffer T (2014) Neuronal plasticity: beyond the critical period. Cell 159:727–737
Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, Straub RE, Ye T, Colantuoni C, Herman MM, Bigelow LB, Weinberger DR, Kleinman JE (2011) Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 31:11088–11095
Ikeda K, Onimaru H, Yamada J, Inoue K, Ueno S, Onaka T, Toyoda H, Arata A, Ishikawa TO, Taketo MM, Fukuda A, Kawakami K (2004) Malfunction of respiratory-related neuronal activity in Na+, K+-ATPase alpha2 subunit-deficient mice is attributable to abnormal Cl- homeostasis in brainstem neurons. J Neurosci 24:10693–10701
Jeyabalan N, Clement JP (2016) SYNGAP1: mind the gap. Front Cell Neurosci 10:32
Kadiyala SB, Papandrea D, Herron BJ, Ferland RJ (2014) Segregation of seizure traits in C57 black mouse substrains using the repeated-flurothyl model. PLoS ONE 9:e90506
Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J (2014) Cation-chloride co-transporters in neuronal development, plasticity and disease. Nat Rev Neurosci 15:637–654
Kepecs A, Fishell G (2014) Interneuron cell types are fit to function. Nature 505:318–326
Kilinc M, Creson T, Rojas C, Aceti M, Ellegood J, Vaissiere T, Lerch JP, Rumbaugh G (2018) Species-conserved SYNGAP1 phenotypes associated with neurodevelopmental disorders. Mol Cell Neurosci 91:140–150
Kimura Y, Akahira-Azuma M, Harada N, Enomoto Y, Tsurusaki Y, Kurosawa K (2018) Novel SYNGAP1 variant in a patient with intellectual disability and distinctive dysmorphisms. Congenit Anom (kyoto) 58:188–190
Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJ, O’Carroll CM, Martin SJ, Morris RG, O’Dell TJ, Grant SG (2002) SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci 22:9721–9732
Larson J, Munkacsy E (2015) Theta-burst LTP. Brain Res 1621:38–50
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T (2013) Object recognition test in mice. Nat Protoc 8:2531–2537
Li H, Khirug S, Cai C, Ludwig A, Blaesse P, Kolikova J, Afzalov R, Coleman SK, Lauri S, Airaksinen MS, Keinanen K, Khiroug L, Saarma M, Kaila K, Rivera C (2007) KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron 56:1019–1033
Liu E, Xie AJ, Zhou Q, Li M, Zhang S, Li S, Wang W, Wang X, Wang Q, Wang JZ (2017) GSK-3beta deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory. Sci Rep 7:5781
Llano O, Smirnov S, Soni S, Golubtsov A, Guillemin I, Hotulainen P, Medina I, Nothwang HG, Rivera C, Ludwig A (2015) KCC2 regulates actin dynamics in dendritic spines via interaction with beta-PIX. J Cell Biol 209:671–686
Lueptow LM (2017) Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. https://doi.org/10.3791/55718
Lugo JN, Swann JW, Anderson AE (2014) Early-life seizures result in deficits in social behavior and learning. Exp Neurol 256:74–80
Madhwal S, Shin M, Kapoor A, Goyal M, Joshi MK, Ur Rehman PM, Gor K, Shim J, Mukherjee T (2020) Metabolic control of cellular immune-competency by odors in Drosophila. Elife 9:e60376
Magee JC, Johnston D (1995) Characterisation of single voltage-gated Na+ and Ca2+ channels in apical dendrites of rat CA1 pyramidal neurons. J Physiol 487:67–90
McCamphill PK, Stoppel LJ, Senter RK, Lewis MC, Heynen AJ, Stoppel DC, Sridhar V, Collins KA, Shi X, Pan JQ, Madison J, Cottrell JR, Huber KM, Scolnick EM, Holson EB, Wagner FF, Bear MF (2020) Selective inhibition of glycogen synthase kinase 3α corrects pathophysiology in a mouse model of fragile X syndrome. Sci Transl Med 12(544):eaam8572. https://doi.org/10.1126/scitranslmed.aam8572
Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, Crovace C, Tarricone C, Musacchio A, Roe SM, Pearl L, Greengard P (2003) GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol 10:1255–1266
Migliore M, Hoffman DA, Magee JC, Johnston D (1999) Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci 7:5–15
Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, Bauchwitz RP (2009) Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology 56:463–472
Mines MA, Jope RS (2011) Glycogen synthase kinase-3: a promising therapeutic target for fragile x syndrome. Front Mol Neurosci 4:35
Muhia M, Yee BK, Feldon J, Markopoulos F, Knuesel I (2010) Disruption of hippocampus-regulated behavioural and cognitive processes by heterozygous constitutive deletion of SynGAP. Eur J Neurosci 31:529–543
Nakajima R, Takao K, Hattori S, Shoji H, Komiyama NH, Grant SGN, Miyakawa T (2019) Comprehensive behavioral analysis of heterozygous Syngap1 knockout mice. Neuropsychopharmacol Rep 39:223–237
Nakao K, Singh M, Sapkota K, Hagler BC, Hunter RN, Raman C, Hablitz JJ, Nakazawa K (2020) GSK3beta inhibition restores cortical gamma oscillation and cognitive behavior in a mouse model of NMDA receptor hypofunction relevant to schizophrenia. Neuropsychopharmacology 45:2207–2218
Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87:2624–2628
Ormond J, Woodin MA (2011) Disinhibition-Mediated LTP in the Hippocampus is Synapse Specific. Front Cell Neurosci 5:17
Owens DF, Boyce LH, Davis MB, Kriegstein AR (1996) Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J Neurosci 16:6414–6423
Ozkan ED, Creson TK, Kramar EA, Rojas C, Seese RR, Babyan AH, Shi Y, Lucero R, Xu X, Noebels JL, Miller CA, Lynch G, Rumbaugh G (2014) Reduced cognition in Syngap1 mutants is caused by isolated damage within developing forebrain excitatory neurons. Neuron 82:1317–1333
Park YY, Johnston D, Gray R (2013) Slowly inactivating component of Na+ current in peri-somatic region of hippocampal CA1 pyramidal neurons. J Neurophysiol 109:1378–1390
Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, Lo E, Wu D, Saule E, Bouschet T, Matthews P, Isaac JT, Bortolotto ZA, Wang YT, Collingridge GL (2007) LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53:703–717
Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14:285–293
Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, Musacchio A, Roe SM, Pearl L, Leost M, Greengard P, Meijer L (2004) Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem 47:935–946
Raveendran VA, Pressey JC, Woodin MA (2020) A novel small molecule targets NKCC1 to restore synaptic inhibition. Trends Pharmacol Sci 41:897–899
Reger ML, Hovda DA, Giza CC (2009) Ontogeny of Rat Recognition Memory measured by the novel object recognition task. Dev Psychobiol 51:672–678
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K (1999) The K+/Cl- co-transporter KCC2 renders GABA hyperpolarising during neuronal maturation. Nature 397:251–255
Rivera C, Voipio J, Kaila K (2005) Two developmental switches in GABAergic signalling: the K+-Cl- co-transporter KCC2 and carbonic anhydrase CAVII. J Physiol 562:27–36
Salcedo-Tello P, Ortiz-Matamoros A, Arias C (2011) GSK3 Function in the brain during development, neuronal plasticity, and neurodegeneration. Int J Alzheimers Dis 2011:189728
Samoriski GM, Applegate CD (1997) Repeated generalised seizures induce time-dependent changes in the behavioral seizure response independent of continued seizure induction. J Neurosci 17:5581–5590
Savardi A, Borgogno M, Narducci R, La Sala G, Ortega JA, Summa M, Armirotti A, Bertorelli R, Contestabile A, De Vivo M, Cancedda L (2020) Discovery of a small molecule drug candidate for selective NKCC1 inhibition in brain disorders. Chem 6:2073–2096
Shim J, Mukherjee T, Banerjee U (2012) Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol 14:394–400
Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K (2005) Depolarising GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarising potentials in the immature hippocampus. J Neurosci 25:5280–5289
Sipila ST, Huttu K, Voipio J, Kaila K (2006) Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarising potentials are driven by a persistent Na+ current and terminated by a slow Ca2+ -activated K+ current. Eur J Neurosci 23:2330–2338
Sullivan BJ, Ammanuel S, Kipnis PA, Araki Y, Huganir RL, Kadam SD (2020) Low-dose perampanel rescues cortical gamma dysregulation associated with parvalbumin interneuron GluA2 upregulation in epileptic Syngap1(+/-) mice. Biol Psychiatry 87:829–842
Suresh SN, Chavalmane AK, Dj V, Yarreiphang H, Rai S, Paul A, Clement JP, Alladi PA, Manjithaya R (2017) A novel autophagy modulator 6-Bio ameliorates SNCA/alpha-synuclein toxicity. Autophagy 13:1221–1234
Tang X, Drotar J, Li K, Clairmont CD, Brumm AS, Sullins AJ, Wu H, Liu XS, Wang J, Gray NS, Sur M, Jaenisch R (2019) Pharmacological enhancement of KCC2 gene expression exerts therapeutic effects on human Rett syndrome neurons and Mecp2 mutant mice. Sci Transl Med 11(503):eaau0164. https://doi.org/10.1126/scitranslmed.aau0164
Tottenham N, Gabard-Durnam LJ (2017) The developing amygdala: a student of the world and a teacher of the cortex. Curr Opin Psychol 17:55–60
Verma V, Paul A, Amrapali Vishwanath A, Vaidya B, Clement JP (2019) Understanding intellectual disability and autism spectrum disorders from common mouse models: synapses to behaviour. Open Biol 9:180265
Vlaskamp DRM, Shaw BJ, Burgess R, Mei D, Montomoli M, Xie H, Myers CT, Bennett MF, XiangWei W, Williams D, Maas SM, Brooks AS, Mancini GMS, Imbh van de Laar JM, van Hagen TL, Ware RI, Webster S, Malone SF, Berkovic RM, Kalnins F, Sicca GC, van Korenke CMA, Ravenswaaij-Arts MS, Hildebrand HC, Mefford Y, Jiang RG, Scheffer IE (2019) SYNGAP1 encephalopathy: a distinctive generalised developmental and epileptic encephalopathy. Neurology 92:e96–e107
Vougogiannopoulou K, Skaltsounis AL (2012) From Tyrian purple to kinase modulators: naturally halogenated indirubins and synthetic analogues. Planta Med 78:1515–1528
Yao HB, Shaw PC, Wong CC, Wan DC (2002) Expression of glycogen synthase kinase-3 isoforms in mouse tissues and their transcription in the brain. J Chem Neuroanat 23:291–297
Acknowledgements
We would like to thank Prof. Ted Abel and Dr Joseph C. Glykys for the critical comments about the concept and the data. We would like to thank Drs. Elizabeth Nicholson, Milos Petrovic, Emin Ozkan, and Jonathan Brown for valuable suggestions on perforated patch experiments. We acknowledge Ginni Khurana and Shruthi Sateesh for their technical inputs in immunoblotting and IHC. We express thanks to Zahid MD for assisting in behavioural experiments. We thank Prof. Vidita Vaidya, Sonali Salvi, Sukrita Deb and other lab members for their technical contributions in mRNA experiments. We thank Suma BS for the technical inputs in confocal imaging. We thank Ankita Kapoor, Drisya Dileep, Lakshmi Balasubramaniam and Shubham Kesarwani for their technical inputs in the analysis of IHC images. We thank RM, Preeti Kute, Bharti Nawalpuri and other lab members for the technical inputs in immunoblotting experiments. We thank Clement lab and Dr Sthitapranjya Pati for all technical and conceptual suggestions towards this manuscript. We thank Prof Upinder Bhalla, Dr Rishikesh Narayanan and Dr Aditi Bhattacharya for their critical comments on this manuscript. Our manuscript was submitted as a pre-print in the Research Square as “Reversing GABA polarity corrects synaptic physiology and behavioural deficits in young adolescent Syngap1+/- mice” (https://doi.org/10.21203/rs.3.rs-682039/v1).
Funding
The work was supported in part by grants to JPC from DST-SERB (SB/YS/LS-215/2013, and EMR/2016/004795), DBT-JNCASR collaborative fund, to RSM in part, by Dept. of Biotechnology, India (BT/PR8723/AGR/36/776/2013, and BT/IN/Denmark/07/RSM/2015-2016), to TB by NSFC (31871076) and intramural funding from all the institutes.
Author information
Authors and Affiliations
Contributions
Electrophysiological recordings, mRNA studies, immunoblotting, immunohistochemistry and behavioural studies were performed by VV; behaviour analysis was done by MJVK; VV, and JPC designed the experiments; TB, and RSM provided technical assistance in ECl− and tonic current experiments, mRNA, immunoblotting studies respectively; KS and SR synthesised and provided 6BIO; JPC conceived the study; VV and JPC wrote, and VV, RSM, RM, TB, and JPC edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethics approval
All the experiments were performed in accordance with the Institutional Animal Ethics Committee (IAEC; Chair: Prof Anuranjan Anand) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Consent to participate
Not applicable.
Content for publication
All authors have given consent to publish this manuscript in the current format.
Additional information
Communicated by Sreedharan Sajikumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vijaya Verma is a joint corresponding author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verma, V., Kumar, M.J.V., Sharma, K. et al. Pharmacological intervention in young adolescents rescues synaptic physiology and behavioural deficits in Syngap1+/− mice. Exp Brain Res 240, 289–309 (2022). https://doi.org/10.1007/s00221-021-06254-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06254-x