Abstract
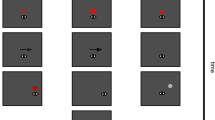
Reduction of retinal speed and alignment of the line of sight are believed to be the respective primary functions of smooth pursuit and saccadic eye movements. As the eye muscles strength can change in the short-term, continuous adjustments of motor signals are required to achieve constant accuracy. While adaptation of saccade amplitude to systematic position errors has been extensively studied, we know less about the adaptive response to position errors during smooth pursuit initiation, when target motion has to be taken into account to program saccades, and when position errors at the saccade endpoint could also be corrected by increasing pursuit velocity. To study short-term adaptation (250 adaptation trials) of tracking eye movements, we introduced a position error during the first catch-up saccade made during the initiation of smooth pursuit—in a ramp-step-ramp paradigm. The target position was either shifted in the direction of the horizontally moving target (forward step), against it (backward step) or orthogonally to it (vertical step). Results indicate adaptation of catch-up saccade amplitude to back and forward steps. With vertical steps, saccades became oblique, by an inflexion of the early or late saccade trajectory. With a similar time course, post-saccadic pursuit velocity was increased in the step direction, adding further evidence that under some conditions pursuit and saccades can act synergistically to reduce position errors.







Similar content being viewed by others
References
Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P (1999) Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19:10931–10939
Blohm G, Missal M, Lefevre P (2005) Direct evidence for a position input to the smooth pursuit system. J Neurophysiol 94:712–721
Bridgeman B, Hendry D, Stark L (1975) Failure to detect displacement of the visual world during saccadic eye movements. Vis Res 15:719–722
Carl JR, Gellman RS (1987) Human smooth pursuit: stimulus-dependent responses. J Neurophysiol 57:1446–1463
Catz N, Dicke PW, Thier P (2008) Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci USA 105:7309–7314
Cavanagh P, Mather G (1989) Motion: the long and short of it. Spat Vis 4:103–129
Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R (2008) Adaptive control of saccades via internal feedback. J Neurosci 28:2804–2813
Churchland AK, Lisberger SG (2002) Gain control in human smooth-pursuit eye movements. J Neurophysiol 87:2936–2945
de Brouwer S, Missal M, Barnes G, Lefevre P (2002a) Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol 87:1772–1780
de Brouwer S, Yuksel D, Blohm G, Missal M, Lefevre P (2002b) What triggers catch-up saccades during visual tracking? J Neurophysiol 87:1646–1650
Desmurget M, Pelisson D, Urquizar C, Prablanc C, Alexander GE, Grafton ST (1998) Functional anatomy of saccadic adaptation in humans. Nat Neurosci 1:524–528
Dodge R (1900) Visual perception during eye movement. Psychol Rev 7:454–465
Edelman JA, Goldberg ME (2002) Effect of short-term saccadic adaptation on saccades evoked by electrical stimulation in the primate superior colliculus. J Neurophysiol 87:1915–1923
Erkelens CJ (2006) Coordination of smooth pursuit and saccades. Vis Res 46:163–170
Ethier V, Zee DS, Shadmehr R (2008) Spontaneous recovery of motor memory during saccade adaptation. J Neurophysiol 99:2577–2583
Frens MA, Van Opstal AJ (1997) Monkey superior colliculus activity during short-term saccadic adaptation. Brain Res Bull 43:473–483
Fuchs AF, Reiner D, Pong M (1996) Transfer of gain changes from targeting to other types of saccade in the monkey: constraints on possible sites of saccadic gain adaptation. J Neurophysiol 76:2522–2535
Fukushima K, Tanaka M, Suzuki Y, Fukushima J, Yoshida T (1996) Adaptive changes in human smooth pursuit eye movement. Neurosci Res 25:391–398
Gellman RS, Carl JR (1991) Motion processing for saccadic eye movements in humans. Exp Brain Res 84:660–667
Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P (2008) Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci 27:132–144
Guan Y, Eggert T, Bayer O, Buttner U (2005) Saccades to stationary and moving targets differ in the monkey. Exp Brain Res 161:220–232
Hafed ZM, Krauzlis RJ (2008) Goal representations dominate superior colliculus activity during extrafoveal tracking. J Neurosci 28:9426–9439
Hafed ZM, Goffart L, Krauzlis RJ (2008) Superior colliculus inactivation causes stable offsets in eye position during tracking. J Neurosci 28:8124–8137
Hallett PE (1978) Primary and secondary saccades to goals defined by instructions. Vis Res 18:1279–1296
Hopp JJ, Fuchs AF (2002) Investigating the site of human saccadic adaptation with express and targeting saccades. Exp Brain Res 144:538–548
Hopp JJ, Fuchs AF (2004) The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol 72:27–53
Ilg UJ (1997) Slow eye movements. Progr Neurobiol 53:293–329
Kahlon M, Lisberger SG (1996) Coordinate system for learning in the smooth pursuit eye movements of monkeys. J Neurosci 16:7270–7283
Kahlon M, Lisberger SG (2000) Changes in the responses of Purkinje cells in the floccular complex of monkeys after motor learning in smooth pursuit eye movements. J Neurophysiol 84:2945–2960
Kaku Y, Yoshida K, Iwamoto Y (2009) Learning signals from the superior colliculus for adaptation of saccadic eye movements in the monkey. J Neurosci 29:5266–5275
Keller EL, Heinen SJ (1991) Generation of smooth-pursuit eye movements: neuronal mechanisms and pathways. Neurosci Res 11:79–107
Kim CE, Thaker GK, Ross DE, Medoff D (1997) Accuracies of saccades to moving targets during pursuit initiation and maintenance. Exp Brain Res 113:371–377
Kimmig HG, Biscaldi M, Mutter J, Doerr JP, Fischer B (2002) The initiation of smooth pursuit eye movements and saccades in normal subjects and in “express-saccade makers”. Exp Brain Res 144:373–384
Kojima Y, Iwamoto Y, Robinson FR, Noto CT, Yoshida K (2008) Premotor inhibitory neurons carry signals related to saccade adaptation in the monkey. J Neurophysiol 99:220–230
Kolers PA (1972) Aspects of motion perception. Pergamon Press, Oxford
Krauzlis RJ (2001) Extraretinal inputs to neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 86:2629–2633
Krauzlis RJ (2003) Neuronal activity in the rostral superior colliculus related to the initiation of pursuit and saccadic eye movements. J Neurosci 23:4333–4344
Krauzlis RJ (2004) Recasting the smooth pursuit eye movement system. J Neurophysiol 91:591–603
Krauzlis RJ (2005) The control of voluntary eye movements: new perspectives. Neuroscientist 11:124–137
Krauzlis RJ, Basso MA, Wurtz RH (1997) Shared motor error for multiple eye movements. Science 276:1693–1695
Krauzlis RJ, Basso MA, Wurtz RH (2000) Discharge properties of neurons in the rostral superior colliculus of the monkey during smooth-pursuit eye movements. J Neurophysiol 84:876–891
Lisberger SG (1998) Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 79:1918–1930
Lisberger SG, Morris EJ, Tychsen L (1987) Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci 10:97–129
Melis BJ, van Gisbergen JA (1996) Short-term adaptation of electrically induced saccades in monkey superior colliculus. J Neurophysiol 76:1744–1758
Munoz DP (2002) Commentary: saccadic eye movements: overview of neural circuitry. Prog Brain Res 140:89–96
Nagao S, Kitazawa H (1998) Adaptive modifications of post-saccadic smooth pursuit eye movements and their interaction with saccades and the vestibulo-ocular reflex in the primate. Neurosci Res 32:157–169
Newsome WT, Wurtz RH, Dursteler MR, Mikami A (1985) Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci 5:825–840
Ogawa T, Fujita M (1997) Adaptive modifications of human postsaccadic pursuit eye movements induced by a step-ramp-ramp paradigm. Exp Brain Res 116:83–96
Optican LM, Robinson DA (1980) Cerebellar-dependent adaptive control of primate saccadic system. J Neurophysiol 44:1058–1076
Optican LM, Zee DS, Chu FC (1985) Adaptive response to ocular muscle weakness in human pursuit and saccadic eye movements. J Neurophysiol 54:110–122
Orban de Xivry JJ, Lefevre P (2007) Saccades and pursuit: two outcomes of a single sensorimotor process. J Physiol 584:11–23
Orban de Xivry JJ, Bennett SJ, Lefevre P, Barnes GR (2006) Evidence for synergy between saccades and smooth pursuit during transient target disappearance. J Neurophysiol 95:418–427
Orban de Xivry JJ, Missal M, Lefevre P (2008) A dynamic representation of target motion drives predictive smooth pursuit during target blanking. J Vis 8(15):6.1–13
Pola J, Wyatt HJ (1980) Target position and velocity: the stimuli for smooth pursuit eye movements. Vis Res 20:523–534
Quessy S, Quinet J, Freedman EG (2010) The locus of motor activity in the superior colliculus of the rhesus monkey is unaltered during saccadic adaptation. J Neurosci 30:14235–14244
Rashbass C (1961) The relationship between saccadic and smooth tracking eye movements. J Physiol 159:326–338
Robinson DA (1965) The mechanics of human smooth pursuit eye movement. J Physiol 180:569–591
Robinson FR, Fuchs AF, Noto CT (2002) Cerebellar influences on saccade plasticity. Ann N Y Acad Sci 956:155–163
Ross J, Morrone MC, Goldberg ME, Burr DC (2001) Changes in visual perception at the time of saccades. Trends Neurosci 24:113–121
Soetedjo R, Fuchs AF (2006) Complex spike activity of purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci 26:7741–7755
Soetedjo R, Kojima Y, Fuchs AF (2008) Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol 100:1949–1966
Straube A, Deubel H (1995) Rapid gain adaptation affects the dynamics of saccadic eye movements in humans. Vis Res 35:3451–3458
Straube A, Deubel H, Ditterich J, Eggert T (2001) Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology 57:2105–2108
Takagi M, Zee DS, Tamargo RJ (1998) Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80:1911–1931
Takagi M, Zee DS, Tamargo RJ (2000) Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol 83:2047–2062
Takeichi N, Kaneko CR, Fuchs AF (2007) Activity changes in monkey superior colliculus during saccade adaptation. J Neurophysiol 97:4096–4107
Thier P, Ilg UJ (2005) The neural basis of smooth-pursuit eye movements. Curr Opin Neurobiol 15:645–652
Wurtz RH (2008) Neuronal mechanisms of visual stability. Vis Res 48:2070–2089
Wurtz RH, Albano JE (1980) Visual-motor function of the primate superior colliculus. Annu Rev Neurosci 3:189–226
Wyatt HJ, Pola J (1987) Smooth eye movements with step-ramp stimuli: the influence of attention and stimulus extent. Vis Res 27:1565–1580
Wyatt HJ, Pola J, Lustgarten M (1989) The oculomotor “twitch”–a transient response to target motion. Exp Brain Res 76:581–592
Xu-Wilson M, Chen-Harris H, Zee DS, Shadmehr R (2009) Cerebellar contributions to adaptive control of saccades in humans. J Neurosci 29:12930–12939
Acknowledgments
Author ACS was supported by the DFG Forschergruppe FOR 560 “Perception and action” and the DFG Graduiertenkolleg GRK 885 “NeuroAct”. Author DS was supported by the Swiss National Science Foundation PBGEP1-125961. We would like to thank Laurent Goffart and Karl Gegenfurtner for advice; Dirk Kerzel for help with programming; Jean-Jacques Orban de Xivry and one anonymous reviewer for constructive criticism as well as Sabine Born and Leily Zarian for their participation as observers. Both authors contributed equally to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schütz, A.C., Souto, D. Adaptation of catch-up saccades during the initiation of smooth pursuit eye movements. Exp Brain Res 209, 537–549 (2011). https://doi.org/10.1007/s00221-011-2581-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2581-7