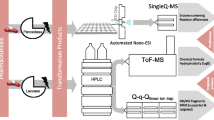
Abstract
Moxidectin (MOX) is a widely used anthelmintic drug for the treatment of internal and external parasites in food-producing and companion animals. Transformation products (TPs) of MOX, formed through metabolic degradation or acid hydrolysis, may pose a potential environmental risk, but only few were identified so far. In this study, we therefore systematically characterized electro- and photochemically generated MOX TPs using high-resolution mass spectrometry (HRMS). Oxidative electrochemical (EC) TPs were generated in an electrochemical reactor and photochemical (PC) TPs by irradiation with UV-C light. Subsequent HRMS measurements were performed to identify accurate masses and deduce occurring modification reactions of derived TPs in a suspected target analysis. In total, 26 EC TPs and 59 PC TPs were found. The main modification reactions were hydroxylation, (de-)hydration, and derivative formation with methanol for EC experiments and isomeric changes, (de-)hydration, and changes at the methoxime moiety for PC experiments. In addition, several combinations of different modification reactions were identified. For 17 TPs, we could predict chemical structures through interpretation of acquired MS/MS data. Most modifications could be linked to two specific regions of MOX. Some previously described metabolic reactions like hydroxylation or O-demethylation were confirmed in our EC and PC experiments as reaction type, but the corresponding TPs were not identical to known metabolites or degradation products. The obtained knowledge regarding novel TPs and reactions will aid to elucidate the degradation pathway of MOX which is currently unknown.

Graphical abstract





Similar content being viewed by others
References
Awasthi A, Razzak M, Al-Kassas R, Harvey J, Garg S. Analytical profile of moxidectin. Profiles Drug Subst Excip Relat Methodol. 2013;38:315–66. https://doi.org/10.1016/B978-0-12-407691-4.00007-1.
Prichard R, Menez C, Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int J Parasitol Drugs Drug Resist. 2012;2:134–53. https://doi.org/10.1016/j.ijpddr.2012.04.001.
Zulalian J, Stout SJ, daCunha AR, Garces T, Miller P. Absorption, tissue distribution, metabolism, and excretion of moxidectin in cattle. J Agric Food Chem. 1994;42(2):381–7. https://doi.org/10.1021/jf00038a028.
Stout SJ, Dacunha AR, Wu SS, Zulalian J, Afzal J. Moxidectin - characterization of cattle, sheep, and rat in-vitro and in-vivo metabolites by liquid-chromatography tandem mass-spectrometry. J Agric Food Chem. 1994;42(2):388–92. https://doi.org/10.1021/jf00038a029.
Afzal J, Stout SJ, daCunha AR, Miller P. Moxidectin: absorption, tissue distribution, excretion, and biotransformation of 14C-labeled moxidectin in sheep. J Agric Food Chem. 1994;42(8):1767–73. https://doi.org/10.1021/jf00044a037.
Alvinerie M, Dupuy J, Eeckhoutte C, Sutra JF, Kerboeuf D. In vitro metabolism of moxidectin in Haemonchus contortus adult stages. Parasitol Res. 2001;87(9):702–4. https://doi.org/10.1007/s004360100408.
Dupuy J, Escudero E, Eeckhoutte C, Sutra J, Galtier P, Alvinerie M. In vitro metabolism of 14C-moxidectin by hepatic microsomes from various species. Vet Res Commun. 2001;25(5):345–54. https://doi.org/10.1023/A:1010686508307.
Afzal J, Burke AB, Batten PL, DeLay RL, Miller P. Moxidectin: metabolic fate and blood pharmacokinetics of 14C-labeled moxidectin in horses. J Agric Food Chem. 1997;45(9):3627–33. https://doi.org/10.1021/jf960981s.
Perez R, Cabezas I, Sutra JF, Galtier P, Alvinerie M. Faecal excretion profile of moxidectin and ivermectin after oral administration in horses. Vet J. 2001;161(1):85–92. https://doi.org/10.1053/tvjl.2000.0521.
Hentz SG, Fernandes MAM, Del Bianchi M, Reyes FGR, de Souza JKG, Giannotti FM, et al. Faecal excretion of moxidectin in lambs and its persistence in different environmental conditions. Small Rumin Res. 2019;174:26–33. https://doi.org/10.1016/j.smallrumres.2019.02.015.
EMA. Moxidectin-containing veterinary medicines used in cattle, sheep and horses EMEA/V/A/116. 2017.
FDA. Cydectin (moxidectin 0.5%) pour-on for cattle. Environmental Assessment Sheet no AG07187-2. 1997:United State Environment Protection Agency.
Awasthi A, Razzak M, Al-Kassas R, Greenwood DR, Harvey J, Garg S. Isolation and characterization of degradation products of moxidectin using LC, LTQ FT-MS, H/D exchange and NMR. Anal Bioanal Chem. 2012;404(8):2203–22. https://doi.org/10.1007/s00216-012-6393-9.
Bletsou AA, Jeon J, Hollender J, Archontaki E, Thomaidis NS. Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. TrAC Trends Anal Chem. 2015;66:32–44. https://doi.org/10.1016/j.trac.2014.11.009.
ABA B, Fogg LA, Blackwell PA, Blackwell P, Kay P, Pemberton EJ, et al. Veterinary medicines in the environment. Reviews of environmental contamination and toxicology. New York: Springer New York; 2004. p. 1–91.
Ml F, Pérez S, Kantiani L, Barceló D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal Chem. 2008;27(11):991–1007. https://doi.org/10.1016/j.trac.2008.09.010.
Bartikova H, Podlipna R, Skalova L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere. 2016;144:2290–301. https://doi.org/10.1016/j.chemosphere.2015.10.137.
Dı́az-Cruz MS, López de Alda MJ, Barceló D. Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. TrAC Trends Anal Chem. 2003;22(6):340–51. https://doi.org/10.1016/S0165-9936(03)00603-4.
Agüera A, Martínez Bueno MJ, Fernández-Alba AR. New trends in the analytical determination of emerging contaminants and their transformation products in environmental waters. Environ Sci Pollut Res. 2013;20(6):3496–515. https://doi.org/10.1007/s11356-013-1586-0.
Pico Y, Barcelo D. Transformation products of emerging contaminants in the environment and high-resolution mass spectrometry: a new horizon. Anal Bioanal Chem. 2015;407(21):6257–73. https://doi.org/10.1007/s00216-015-8739-6.
Kotthoff L, Keller J, Lörchner D, Mekonnen TF, Koch M. Transformation products of organic contaminants and residues—overview of current simulation methods. Molecules. 2019;24(4). https://doi.org/10.3390/molecules24040753.
Jahn S, Karst U. Electrochemistry coupled to (liquid chromatography/) mass spectrometry--current state and future perspectives. J Chromatogr A. 2012;1259:16–49. https://doi.org/10.1016/j.chroma.2012.05.066.
Portychova L, Schug KA. Instrumentation and applications of electrochemistry coupled to mass spectrometry for studying xenobiotic metabolism: a review. Anal Chim Acta. 2017;993:1–21. https://doi.org/10.1016/j.aca.2017.08.050.
Bruins AP. An overview of electrochemistry combined with mass spectrometry. TrAC Trends Anal Chem. 2015;70:14–9. https://doi.org/10.1016/j.trac.2015.02.016.
Hoffmann T, Hofmann D, Klumpp E, Küppers S. Electrochemistry-mass spectrometry for mechanistic studies and simulation of oxidation processes in the environment. Anal Bioanal Chem. 2011;399(5):1859–68. https://doi.org/10.1007/s00216-010-4575-x.
Lohmann W, Karst U. Simulation of the detoxification of paracetamol using on-line electrochemistry/liquid chromatography/mass spectrometry. Anal Bioanal Chem. 2006;386(6):1701–8. https://doi.org/10.1007/s00216-006-0801-y.
Fatta-Kassinos D, Vasquez MI, Kummerer K. Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes - degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere. 2011;85(5):693–709. https://doi.org/10.1016/j.chemosphere.2011.06.082.
Team RC. R: a language and environment for statistical computing. 2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1121 kb)
Rights and permissions
About this article
Cite this article
Kotthoff, L., O’Callaghan, SL., Lisec, J. et al. Structural annotation of electro- and photochemically generated transformation products of moxidectin using high-resolution mass spectrometry. Anal Bioanal Chem 412, 3141–3152 (2020). https://doi.org/10.1007/s00216-020-02572-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02572-1