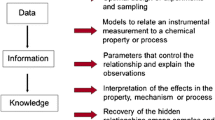
Abstract
The contribution of chemometrics to important stages throughout the entire analytical process such as experimental design, sampling, and explorative data analysis, including data pretreatment and fusion, was described in the first part of the tutorial “Chemometrics in analytical chemistry.” This is the second part of a tutorial article on chemometrics which is devoted to the supervised modeling of multivariate chemical data, i.e., to the building of calibration and discrimination models, their quantitative validation, and their successful applications in different scientific fields. This tutorial provides an overview of the popularity of chemometrics in analytical chemistry.





Similar content being viewed by others
References
Brereton RG, Jansen J, Lopes J, Marini F, Pomerantsev A, Rodionova O, et al. Chemometrics in analytical chemistry—part I: history, experimental design and data analysis tools. Anal Bioanal Chem. 2017;409:5891–9.
Kalivas JH, Calibration Methodologies in Comprehensive Chemometrics, Brown S, Tauler R, Walczak B (Eds.). Amsterdam:Elsevier; 2009, Vol.3, chapter 3.01.
Belsley DA, Kuh E, Welsch RE. Identifying influential data and sources of collinearity. New York: John Wiley & Sons; 1980.
Brereton RG. One Class Classifiers. J Chemometr. 2011;25:225–46.
Wold S, Sjostrom M. SIMCA: a method for analyzing chemical data in terms of similarity and analogy, in Kowalski, BR (Ed) Chemometrics Theory and Application, American Chemical Society Symposium Series 52, Wash., D.C.:American Chemical Society; 1977, 243–282.
Pomerantsev A, OYe R. Concept and role of extreme objects in PCA/SIMCA. J Chemometr. 2014;28:429–38.
Fisher RA. The use of multiple measurements in taxonomic problems. Ann Eugenics. 1936;1936:179M.
Barker M, Rayens W. Partial least squares for discrimination. J Chemom. 2003;17:166–73.
Brereton RG, Lloyd GR. Partial least squares discriminant analysis: taking the magic away. J Chemom. 2014;28:221–35.
Rodionova YO, Titova AV, Pomerantsev AL. Discriminant analysis is an inappropriate method of authentication TRAC trends. Anal Chem. 2016;78(4):17–22.
Anderssen E, Dyrstad K, Westad F, Martens H. Reducing over-optimism in variable selection by cross-model validation Chemomet. Intell Lab Syst. 2006;84:69–74.
Centner V, Massart DL, de Noord OE, de Jong S, Vandeginste B. Sterna C. Anal Chem. 1996;68:3851–8.
Serneels S, Filzmoser P, Croux C, Van Espen PJ. Chemometr Intell Lab Syst. 2005;76:197–204.
Zerzucha P, Walczak B. Concept of (dis)similarity in data analysis TRAC trends. Anal Chem. 2012;38:116–28.
Harshman R. How can I know if it's real? A catalogue of diagnostics for use with three-mode factor analysis and multidimensional scaling. In: Law HG, Snyder Jr CW, Hattie J, Mc Donald RP, editors. Research Methods for Multimode Data Analysis. New York: Praeger; 1984. p. 566–91.
Westad F, Marini F. Validation of chemometric models—a tutorial. Anal Chim Acta. 2015;893:14–24.
Booksh KS, Kowalski BR. Theory of analytical chemistry. Anal Chem. 1994;66(15):782A–91A.
Forina M, Lanteri S, Armarino C. Chemometrics in food chemistry, in Chemometrics and species identification. Berlin: Springer; 1987. p. 91–143.
Kelly JJ, Barlow CH, Jinguji TM, Callis JB. Ana Chem 1989;61(4);313–320.
Wise BM, Gallagher NB. J Process Contr 1996;6(6);329–348.
Sharaf MA, Illman DL, Kowalski BR. Chemometrics, chemical analysis, vol. 82. New York: John Wiley and Sons; 1986.
Hopke PK. Receptor Modling in Environmental Chemistry, New York: John Wiley Sons; 1981; Hopke PK. Modeling for air quality management, Amsterdam:Elsevier; 1991.
Eriksson L, Johansson E. Multivariate design and modeling in QSAR. Chemometr Intell Lab. 1996;34:1–19.
Eriksson L, Byrne T, Johansson E, Trygg J, Wikström C. Multi- and megavariate data analysis basic principles and applications, Umeå. 3rd ed. Sweden: Umetrics academy; 2013.
Parastar H, Tauler R. Big (bio)chemical data mining using Chemometric methods: a need for chemists. Angew Chem Int. 2018; https://doi.org/10.1002/anie.201801134.
Cao K, Lê Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. Bioinformatics. 2011;12:253.
Smilde AK, Jansen JJ, Hoefsloot HCJ, Lamers RJAN, van der Greef J, Timmerman ME. ANOVA-simultaneous component analysis (ASCA): a new tool for analyzing designed metabolomics data. Bioinformatics. 2005;21:3043–8.
van den Berg RA, Hoefsloot HCJ, Westerhuis JA, Smilde AK, van der Werf MJ. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 2006;7:142.
Gorrochategui E, Jaumot J, Lacorte S, Tauler R. Data analysis strategies for targeted and untargeted LC-MS metabolomic studies: overview and workflow. TrAC - Trends in Anal Chem. 2016;82:425–42.
Grahn HF, Geladi P, editors. Techniques and applications of hyperspectral image analysis. Chichester, UK: John Wiley & Sons Ltd; 2005.
Geladi P, Grahn H. Multivariate image analysis in chemistry and related areas: chemometric image analysis. Chichester UK: Wiley; 1996.
Olmos V, Benítez L, Marro M, Loza-Alvarez P, Piña B, Tauler R, et al. Relevant aspects of unmixing/resolution analysis for the interpretation of biological vibrational hyperspectral images. TrAC-Trends in Anal Chem. 2017;94:130–40.
Felten J, Hall H, Jaumot J, Tauler R, de Juan A, Gorzsás A. Vibrational spectroscopic image analysis of biological material using multivariate curve resolution–alternating least squares (MCR-ALS). Nat Protoc. 2015;10:217–40.
Piqueras S, Bedia C, Beleites C, Krafft C, Popp J, Maeder M, et al. Handling different spatial resolution in image fusion by multivariate curve resolution-alternating least squares for incomplete image multisets. Anal Chem. 2018;90(11):6757–65.
Setou M. (Ed.) Imaging mass spectrometry. Protocols for Mass Microscopy, Berlin:Springer; 2010.
Rubakhin SS, Sweedler JV (Eds), mass spectrometry imaging. Principles and protocols. New York: Humana Press; 2010.
Bedia C, Tauler R, Jaumot J. Compression strategies for the chemometric analysis of mass spectrometry imaging data. J Chemom. 2016;30:575–88.
Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of analytical chemistry. Ninth ed. Belmont, CA: Brooks/Cole; 2014.
Christian GD, Dasgupta PN, Schug KA. Analytical chemistry. seventh ed. New York: Wiley; 2013.
Zomaya AY, Sakr S. Handbook of big data technologies. Berlin: Springer; 2017.
Burges CJC. A tutorial on support vector machines for pattern recognition. Data Min Know Disc. 1998;2:121–67.
Kohonen T. Self-Organizing maps. Third ed. Berlin: Springer; 2001.
Schmidhuber J. Deep learning in neural networks: an overview http://arxiv.org/abs/1404.7828, 2014.
Lutsa J, Ojedaa F, Van de Plasa R, De Moora B, Van Huffel S, Suykens JAK. A tutorial on support vector machine-based methods for classification problems in chemometrics. Anal Chim Acta. 2010;665:129–45.
Nia W, Nørgaard L, Mørup M. Non-linear calibration models for near infrared spectroscopy. Anal Chim Acta. 2014;813:1–14.
Thissen U, Pepers M, Ustun B, Melssen WJ, Buydens LMC. Comparing support vector machines to PLS for spectral regression applications. Chemometr Intell Lab Syst. 2004;73:169–79.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Brereton, R.G., Jansen, J., Lopes, J. et al. Chemometrics in analytical chemistry—part II: modeling, validation, and applications. Anal Bioanal Chem 410, 6691–6704 (2018). https://doi.org/10.1007/s00216-018-1283-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1283-4