Abstract
Dosage adjustment of anti-epileptic drugs by therapeutic drug monitoring (TDM) is very useful, especially for the first-generation anti-epileptic drugs (AEDs). Microsampling—the collection of small volumes of blood—is increasingly considered a valuable alternative to conventional venous sampling for TDM. Volumetric absorptive microsampling (VAMS) allows accurate and precise collection of a fixed volume of blood, eliminating the volumetric blood hematocrit bias coupled to conventional dried blood spot collection. The aim of this study was to develop and validate an LC-MS/MS method for the determination and quantification of four anti-epileptic drugs (carbamazepine, valproic acid, phenobarbital, and phenytoin) and one active metabolite (carbamazepine-10,11-epoxide) in samples collected by VAMS. The method was fully validated based on international guidelines. Precision (%RSD) was below 10%, while, with a single exception, accuracy (%bias) met the acceptance criteria. Neither carry-over nor unacceptable interferences were observed, the method being able to distinguish between the isomers oxcarbazepine and carbamazepine-10,11-epoxide. All compounds were stable in VAMS samples for at least 1 month when stored at room temperature, 4 °C, and − 20 °C and for at least 1 week when stored at 60 °C. Internal standard-corrected matrix effects were below 10%, with %RSDs below 4%. High (> 85%) recovery values were obtained and the effect of the hematocrit on the recovery was overall limited. Successful application on external quality control materials and on left-over patient samples demonstrated the validity and applicability of the developed procedure.

Graphical representation of the sampling, chemical structures, and the resulting chromatogram for volumetric absorptive microsampling (VAMS)-based therapeutic drug monitoring of first-generation anti-epileptic drugs by liquid chromatography with tandem mass spectrometric detection.




Similar content being viewed by others
Change history
15 February 2018
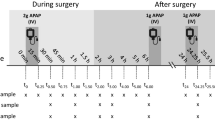
We would like to call the reader’s attention to the fact that unfortunately in fig. 2 of the original article the figure headings of both graphs are the same.
References
Milosheska D, Grabnar I, Vovk T. Dried blood spots for monitoring and individualization of antiepileptic drug treatment. Eur J Pharm Sci. 2015;75:25–39.
Krasowski MD, McMillin GA. Advances in anti-epileptic drug testing. Clin Chim Acta. 2014;436:224–36.
Velghe S, Capiau S, Stove CP. Opening the toolbox of alternative sampling strategies in clinical routine: a key-role for (LC-)MS/MS. Trac Trend Anal Chem. 2016;84:61–73.
Capiau S, Alffenaar J-W, Stove CP. Alternative sampling strategies for therapeutic drug monitoring. In: Clarke W, Dasgupta A, editors. Clinical challenges in therapeutic drug monitoring. Amsterdam: Elsevier; 2016. p. 279–336.
Shah NM, Hawwa AF, Millership JS, Collier PS, McElnay JC. A simple bioanalytical method for the quantification of antiepileptic drugs in dried blood spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;923-924:65–73.
Popov TV, Maricic LC, Prosen H, Voncina DB. Development and validation of dried blood spots technique for quantitative determination of topiramate using liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2013;27(8):1054–61.
Linder C, Andersson M, Wide K, Beck O, Pohanka A. A LC-MS/MS method for therapeutic drug monitoring of carbamazepine, lamotrigine and valproic acid in DBS. Bioanalysis. 2015;7(16):2031–9.
Villanelli F, Giocaliere E, Malvagia S, Rosati A, Forni G, Funghini S, et al. Dried blood spot assay for the quantification of phenytoin using liquid chromatography-mass spectrometry. Clin Chim Acta. 2015;440:31–5.
Shokry E, Villanelli F, Malvagia S, Rosati A, Forni G, Funghini S, et al. Therapeutic drug monitoring of carbamazepine and its metabolite in children from dried blood spots using liquid chromatography and tandem mass spectrometry. J Pharm Biomed Anal. 2015;109:164–70.
AbuRuz S, Al-Ghazawi M, Al-Hiari Y. A simple dried blood spot assay for therapeutic drug monitoring of lamotrigine. Chromatographia. 2010;71(11–12):1093–9.
la Marca G, Malvagia S, Filippi L, Luceri F, Moneti G, Guerrini R. A new rapid micromethod for the assay of phenobarbital from dried blood spots by LC-tandem mass spectrometry. Epilepsia. 2009;50(12):2658–62.
la Marca G, Malvagia S, Filippi L, Fiorini P, Innocenti M, Luceri F, et al. Rapid assay of topiramate in dried blood spots by a new liquid chromatography-tandem mass spectrometric method. J Pharm Biomed Anal. 2008;48(5):1392–6.
Wilhelm AJ, den Burger JC, Swart EL. Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet. 2014;53(11):961–73.
Fan L, Lee JA. Managing the effect of hematocrit on DBS analysis in a regulated environment. Bioanalysis. 2012;4(4):345–7.
De Kesel PM, Capiau S, Lambert WE, Stove CP. Current strategies for coping with the hematocrit problem in dried blood spot analysis. Bioanalysis. 2014;6(14):1871–4.
Youhnovski N, Bergeron A, Furtado M, Garofolo F. Pre-cut dried blood spot (PCDBS): an alternative to dried blood spot (DBS) technique to overcome hematocrit impact. Rapid Commun Mass Spectrom. 2011;25(19):2951–8.
De Kesel PM, Capiau S, Stove VV, Lambert WE, Stove CP. Potassium-based algorithm allows correction for the hematocrit bias in quantitative analysis of caffeine and its major metabolite in dried blood spots. Anal Bioanal Chem. 2014;406(26):6749–55.
den Burger JC, Wilhelm AJ, Chahbouni AC, Vos RM, Sinjewel A, Swart EL. Haematocrit corrected analysis of creatinine in dried blood spots through potassium measurement. Anal Bioanal Chem. 2015;407(2):621–7.
Li Y, Henion J, Abbott R, Wang P. The use of a membrane filtration device to form dried plasma spots for the quantitative determination of guanfacine in whole blood. Rapid Commun Mass Spectrom. 2012;26(10):1208–12.
Li F, Zulkoski J, Fast D, Michael S. Perforated dried blood spots: a novel format for accurate microsampling. Bioanalysis. 2011;3(20):2321–33.
Meesters RJ, Zhang J, van Huizen NA, Hooff GP, Gruters RA, Luider TM. Dried matrix on paper disks: the next generation DBS microsampling technique for managing the hematocrit effect in DBS analysis. Bioanalysis. 2012;4(16):2027–35.
Leuthold LA, Heudi O, Deglon J, Raccuglia M, Augsburger M, Picard F, et al. New microfluidic-based sampling procedure for overcoming the hematocrit problem associated with dried blood spot analysis. Anal Chem. 2015;87(4):2068–71.
De Kesel PM, Lambert WE, Stove CP. Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Anal Chim Acta. 2015;881:65–73.
Denniff P, Spooner N. Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Anal Chem. 2014;86(16):8489–95.
Verougstraete N, Lapauw B, Van Aken S, Delanghe J, Stove C, Stove V. Volumetric absorptive microsampling at home as an alternative tool for the monitoring of HbA1c in diabetes patients. Clin Chem Lab Med. 2017;55(3):462–9.
Kok MGM, Fillet M. Volumetric absorptive microsampling: current advances and applications. J Pharm Biomed Anal. 2018;147:288–96.
Atugonza R, Kakooza-Mwesige A, Lhatoo S, Kaddumukasa M, Mugenyi L, Sajatovic M, et al. Multiple anti-epileptic drug use in children with epilepsy in Mulago hospital, Uganda: a cross sectional study. BMC Pediatr. 2016;16:34.
Gogtay NJ, Kshirsagar NA, Dalvi SS. Therapeutic drug monitoring in a developing country: an overview. Br J Clin Pharmacol. 2001;52(Suppl 1):103S–8S.
Bertilsson L. Clinical pharmacokinetics of carbamazepine. Clin Pharmacokinet. 1978;3(2):128–43.
U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research. Center for Veterinary Medicine. Draft Guidance for Industry. Bioanalytical Method Validation. 2013. https://www.fda.gov/downloads/Drugs/Guidances/ucm368107.pdf. Accessed June 2017.
European Medicines Agency. Guideline on Bioanalytical Method Validation. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed June 2017.
Wille SMR, Peters FT, Di Fazio V, Samyn N. Practical aspects concerning validation and quality control for forensic and clinical bioanalytical quantitative methods. Accred Qual Assur. 2011;16(6):279–92.
Abu-Rabie P, Denniff P, Spooner N, Chowdhry BZ, Pullen FS. Investigation of different approaches to incorporating internal standard in DBS quantitative bioanalytical workflows and their effect on nullifying hematocrit-based assay bias. Anal Chem. 2015;87(9):4996–5003.
Houts T. Immunochromatography. In: Price CP, editor. Principles and practice of immunoassay. New York: Stockton Press; 1991. p. 563–83.
Launiainen T, Ojanpera I. Drug concentrations in post-mortem femoral blood compared with therapeutic concentrations in plasma. Drug Test Anal. 2014;6(4):308–16.
Morris RG, Schapel GJ. Phenytoin and phenobarbital assayed by the ACCULEVEL method compared with EMIT in an outpatient clinic setting. Ther Drug Monit. 1988;10(4):469–73.
Linder C, Wide K, Walander M, Beck O, Gustafsson LL, Pohanka A. Comparison between dried blood spot and plasma sampling for therapeutic drug monitoring of antiepileptic drugs in children with epilepsy: a step towards home sampling. Clin Biochem. 2017;50(7–8):418–24.
Kong ST, Lim SH, Lee WB, Kumar PK, Wang HY, Ng YL, et al. Clinical validation and implications of dried blood spot sampling of carbamazepine, valproic acid and phenytoin in patients with epilepsy. PLoS One. 2014;9(9):e108190.
Rhoden L, Antunes MV, Hidalgo P, Alvares da Silva C, Linden R. Simple procedure for determination of valproic acid in dried blood spots by gas chromatography-mass spectrometry. J Pharm Biomed Anal. 2014;96:207–12.
Acknowledgements
The authors wish to acknowledge Prof. Veronique Stove, PharmD. Matthijs Oyaert and their team for assistance with blood collection and all volunteers who participated in the study. Furthermore, SV would also like to thank the Special Research Fund (BOF) for granting her a PhD fellowship (application number 01D42414).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Approval for this study was provided by the Ethics Committee of Ghent University Hospital (EC2017/0572). For the use of left-over samples to evaluate an alternative procedure for AED monitoring, the need to obtained individual informed consent was waived by the Ethics Committee.
Conflict of interest
The author declares to not have any financial, commercial, legal, or professional relationship with other organizations, or with the people working with them, that could influence the matter discussed in this manuscript.
Additional information
The original version of this article was revised: the headings of figure 2 of both graphs are the same.
A correction to this article is available online at https://doi.org/10.1007/s00216-018-0951-8.
Electronic supplementary material
ESM 1
(PDF 345 kb)
Rights and permissions
About this article
Cite this article
Velghe, S., Stove, C.P. Volumetric absorptive microsampling as an alternative tool for therapeutic drug monitoring of first-generation anti-epileptic drugs. Anal Bioanal Chem 410, 2331–2341 (2018). https://doi.org/10.1007/s00216-018-0866-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0866-4