Abstract
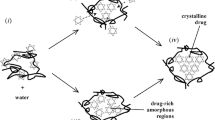
Newly developed active pharmaceutical ingredients (APIs) are often poorly soluble in water. As a result the bioavailability of the API in the human body is reduced. One approach to overcome this restriction is the formulation of amorphous solid dispersions (ASDs), e.g., by hot-melt extrusion (HME). Thus, the poorly soluble crystalline form of the API is transferred into a more soluble amorphous form. To reach this aim in HME, the APIs are embedded in a polymer matrix. The resulting amorphous solid dispersions may contain small amounts of residual crystallinity and have the tendency to recrystallize. For the controlled release of the API in the final drug product the amount of crystallinity has to be known. This review assesses the available analytical methods that have been recently used for the characterization of ASDs and the quantification of crystalline API content. Well-established techniques like near- and mid-infrared spectroscopy (NIR and MIR, respectively), Raman spectroscopy, and emerging ones like UV/VIS, terahertz, and ultrasonic spectroscopy are considered in detail. Furthermore, their advantages and limitations are discussed with regard to general practical applicability as process analytical technology (PAT) tools in industrial manufacturing. The review focuses on spectroscopic methods which have been proven as most suitable for in-line and on-line process analytics. Further aspects are spectroscopic techniques that have been or could be integrated into an extruder.






Similar content being viewed by others
References
Patil H, Tiwari RV, Repka MA. Hot-melt extrusion: from theory to application in pharmaceutical formulation. AAPS PharmSciTech. 2016;17(1):20–42. doi:10.1208/s12249-015-0360-7.
Martin C. Twin screw extruders as continuous mixers for thermal processing: a technical and historical perspective. AAPS PharmSciTech. 2016;17(1):3–19. doi:10.1208/s12249-016-0485-3.
Agrawal A, Dudhedia M, Deng W, Shepard K, Zhong L, Povilaitis E. Development of tablet formulation of amorphous solid dispersions prepared by hot melt extrusion using quality by design approach. AAPS PharmSciTech. 2016;17(1):214–32. doi:10.1208/s12249-015-0472-0.
Agrawal AM, Dudhedia MS, Zimny E. Hot melt extrusion: development of an amorphous solid dispersion for an insoluble drug from mini-scale to clinical scale. AAPS PharmSciTech. 2016;17(1):133–47. doi:10.1208/s12249-015-0425-7.
Rantanen J, Khinast J. The future of pharmaceutical manufacturing sciences. J Pharm Sci. 2015;104(11):3612–38. doi:10.1002/jps.24594.
Schultheiss N, Newman A. Pharmaceutcial cocrystals and their physicochemical properties. Cryst Growth Des. 2009;9(6):2950–67.
Haleblian JK. Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J Pharm Sci. 1975;64(8):1269–88.
Karpinski PH. Polymorphism of active pharmaceutical ingredients. Chem Eng Technol. 2006;29(2):233–7. doi:10.1002/ceat.200500397.
Sala S, Córdoba A, Moreno-Calvo E, Elizondo E, Muntó M, Rojas PE, et al. Crystallization of microparticulate pure polymorphs of active pharmaceutical ingredients using CO2-expanded solvents. Cryst Growth Des. 2012;12(4):1717–26. doi:10.1021/cg200356x.
Li W, Worosila GD, Wang W, Mascaro T. Determination of polymorph conversion of an active pharmaceutical ingredient in wet granulation using NIR calibration models generated from the premix blends. J Pharm Sci. 2005;94(12):2800–6. doi:10.1002/jps.20501.
Lu J, Rohani S. Polymorphism and crystallization of active pharmaceutical ingredients (APIs). Curr Med Chem. 2009;16:884–905.
Virtanen T, Maunu SL. Quantitation of a polymorphic mixture of an active pharmaceutical ingredient with solid state (13)C CPMAS NMR spectroscopy. Int J Pharm. 2010;394(1-2):18–25. doi:10.1016/j.ijpharm.2010.04.017.
Hancock BC, Zografi G. Characteristics and significance of the amorphous state in pharmaceutical systems. J Pharm Sci. 1997;86(1):1–12. doi:10.1021/js9601896.
Lee SL, O’Connor TF, Yang X, Cruz CN, Chatterjee S, Madurawe RD, et al. Modernizing pharmaceutical manufacturing: from batch to continuous production. J Pharm Innov. 2015;10(3):191–9. doi:10.1007/s12247-015-9215-8.
Fonteyne M, Vercruysse J, De Leersnyder F, Van Snick B, Vervaet C, Remon JP, et al. Process analytical technology for continuous manufacturing of solid-dosage forms. Trends Anal Chem. 2015;67:159–66. doi:10.1016/j.trac.2015.01.011.
Simon LL, Pataki H, Marosi G, Meemken F, Hungerbühler K, Baiker A, et al. Assessment of recent process analytical technology (PAT) trends: a multiauthor review. Org Process Res Dev. 2015;19(1):3–62. doi:10.1021/op500261y.
Netchacovitch L, Thiry J, De Bleye C, Chavez PF, Krier F, Sacre PY, et al. Vibrational spectroscopy and microspectroscopy analyzing qualitatively and quantitatively pharmaceutical hot melt extrudates. J Pharm Biomed Anal. 2015;113:21–33. doi:10.1016/j.jpba.2015.01.051.
Erxleben A. Application of vibrational spectroscopy to study solid-state transformations of pharmaceutcials. Curr Pharm Des. 2016;22(33):4883–911. doi:10.2174/138161282266616072.
Challa S, Potumarthi R. Chemometrics-based process analytical technology (PAT) tools: applications and adaptation in pharmaceutical and biopharmaceutical industries. Appl Biochem Biotechnol. 2013;169(1):66–76. doi:10.1007/s12010-012-9950-y.
Food and Drug Administration. Guidance for industry PAT: a framework for innovative pharmaceutcial development, manufacturing, and quality assurance. Rockville: FDA; 2004.
Saerens L, Vervaet C, Remon JP, De Beer T. Process monitoring and visualization solutions for hot-melt extrusion: a review. J Pharm Pharmacol. 2014;66(2):180–203. doi:10.1111/jphp.12123.
Kessler RW. Prozessanalytik Strategien und Fallbeispiele aus der industriellen Praxis. Weinheim: Wiley-VCH; 2006.
Jamrogiewicz M. Application of the near-infrared spectroscopy in the pharmaceutical technology. J Pharm Biomed Anal. 2012;66:1–10. doi:10.1016/j.jpba.2012.03.009.
Luypaert J, Massart DL, Vander Heyden Y. Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta. 2007;72(3):865–83. doi:10.1016/j.talanta.2006.12.023.
Räsänen E, Sandler N. Near infrared spectroscopy in the development of solid dosage forms. J Pharm Pharmacol. 2007;59(2):147–59. doi:10.1211/jpp.59.2.0002.
Reich G. Near-infrared spectroscopy and imaging: basic principles and pharmaceutical applications. Adv Drug Deliv Rev. 2005;57(8):1109–43. doi:10.1016/j.addr.2005.01.020.
Tumuluri SV, Prodduturi S, Crowley MM, Stodghill SP, McGinity JW, Repka MA, et al. The use of near-infrared spectroscopy for the quantitation of a drug in hot-melt extruded films. Drug Dev Ind Pharm. 2004;30(5):505–11. doi:10.1081/DDC-120037481.
Fischer D, Sahre K, Abdelrhim M, Voit B, Sadhu VB, Pionteck J, et al. Process monitoring of polymers by in-line ATR-IR, NIR and Raman spectroscopy and ultrasonic measurements. C R Chim. 2006;9(11-12):1419–24. doi:10.1016/j.crci.2006.06.006.
Roggo Y, Chalus P, Maurer L, Lema-Martinez C, Edmond A, Jent N. A review of near infrared spectroscopy and chemometrics in pharmaceutical technologies. J Pharm Biomed Anal. 2007;44(3):683–700. doi:10.1016/j.jpba.2007.03.023.
Apruzzese F, Pato J, Balke ST, Diosady LL. In-line measurement of residence time distribution in a co-rotating twin-screw extruder. Food Res Int. 2003;36(5):461–7. doi:10.1016/s0963-9969(02)00193-x.
Wahl PR, Treffer D, Mohr S, Roblegg E, Koscher G, Khinast JG. Inline monitoring and a PAT strategy for pharmaceutical hot melt extrusion. Int J Pharm. 2013;455(1-2):159–68. doi:10.1016/j.ijpharm.2013.07.044.
Esbensen KH, Paasch-Mortensen P. Process sampling theory of sampling - the missing link in process analytical technologies (PAT). In: Bakeev KA, editor. Process analytical technology. Chichester: Wiley; 2010. p. 37–80.
Kelly AL, Halsey SA, Bottom RA, Korde S, Gough T, Paradkar A. A novel transflectance near infrared spectroscopy technique for monitoring hot melt extrusion. Int J Pharm. 2015;496(1):117–23. doi:10.1016/j.ijpharm.2015.07.025.
Almeida A, Saerens L, De Beer T, Remon JP, Vervaet C. Upscaling and in-line process monitoring via spectroscopic techniques of ethylene vinyl acetate hot-melt extruded formulations. Int J Pharm. 2012;439(1-2):223–9. doi:10.1016/j.ijpharm.2012.09.037.
Saerens L, Dierickx L, Quinten T, Adriaensens P, Carleer R, Vervaet C, et al. In-line NIR spectroscopy for the understanding of polymer-drug interaction during pharmaceutical hot-melt extrusion. Eur J Pharm Biopharm. 2012;81(1):230–7. doi:10.1016/j.ejpb.2012.01.001.
De Beer T, Burggraeve A, Fonteyne M, Saerens L, Remon JP, Vervaet C. Near infrared and Raman spectroscopy for the in-process monitoring of pharmaceutical production processes. Int J Pharm. 2011;417(1-2):32–47. doi:10.1016/j.ijpharm.2010.12.012.
Maniruzzaman M, Islam MT, Halsey S, Amin D, Douroumis D. Novel controlled release polymer-lipid formulations processed by hot melt extrusion. AAPS PharmSciTech. 2016;17(1):191–9. doi:10.1208/s12249-015-0470-2.
Krier F, Mantanus J, Sacre PY, Chavez PF, Thiry J, Pestieau A, et al. PAT tools for the control of co-extrusion implants manufacturing process. Int J Pharm. 2013;458(1):15–24. doi:10.1016/j.ijpharm.2013.09.040.
Islam MT, Scoutaris N, Maniruzzaman M, Moradiya HG, Halsey SA, Bradley MS, et al. Implementation of transmission NIR as a PAT tool for monitoring drug transformation during HME processing. Eur J Pharm Biopharm. 2015;96:106–16. doi:10.1016/j.ejpb.2015.06.021.
Baronsky-Probst J, Moltgen CV, Kessler W, Kessler RW. Process design and control of a twin screw hot melt extrusion for continuous pharmaceutical tamper-resistant tablet production. Eur J Pharm Sci. 2016;87:14–21. doi:10.1016/j.ejps.2015.09.010.
Kelly AL, Gough T, Dhumal RS, Halsey SA, Paradkar A. Monitoring ibuprofen-nicotinamide cocrystal formation during solvent free continuous cocrystallization (SFCC) using near infrared spectroscopy as a PAT tool. Int J Pharm. 2012;426(1-2):15–20. doi:10.1016/j.ijpharm.2011.12.033.
Siesler HW. Vibrational spectroscopy. Polym Sci. 2012;2:255–300. doi:10.1016/b978-0-444-53349-4.00026-1.
Haberstroh E, Jakisch L, Henßge E, Schwarz P. Real-time monitoring of reactive extrusion processes by means of in-line infrared spectroscopy and infrared temperature measurement. Macromol Mater Eng. 2002;287:203–8.
Coates PD, Barnes SE, Sibley MG, Brown EC, Edwards HGM, Scowen IJ. In-process vibrational spectroscopy and ultrasound measurements in polymer melt extrusion. Polymer. 2003;44(19):5937–49. doi:10.1016/s0032-3861(03)00544-5.
Dong Z, Chatterji A, Sandhu H, Choi DS, Chokshi H, Shah N. Evaluation of solid state properties of solid dispersions prepared by hot-melt extrusion and solvent co-precipitation. Int J Pharm. 2008;355(1-2):141–9. doi:10.1016/j.ijpharm.2007.12.017.
Alshahrani SM, Lu W, Park JB, Morott JT, Alsulays BB, Majumdar S, et al. Stability-enhanced hot-melt extruded amorphous solid dispersions via combinations of Soluplus(R) and HPMCAS-HF. AAPS PharmSciTech. 2015;16(4):824–34. doi:10.1208/s12249-014-0269-6.
Pudlas M, Kyeremateng SO, Williams LA, Kimber JA, van Lishaut H, Kazarian SG, et al. Analyzing the impact of different excipients on drug release behavior in hot-melt extrusion formulations using FTIR spectroscopic imaging. Eur J Pharm Sci. 2015;67:21–31. doi:10.1016/j.ejps.2014.10.012.
Maier H-G. Lebensmittel- und Umweltanalytik: Methoden und Anwendungen. Steinkopff Verlag: Darmstadt; 1990.
De Beer TRM, Bodson C, Dejaegher B, Walczak B, Vercruysse P, Burggraeve A, et al. Raman spectroscopy as a process analytical technology (PAT) tool for the in-line monitoring and understanding of a powder blending process. J Pharm Biomed Anal. 2008;48(3):772–9. doi:10.1016/j.jpba.2008.07.023.
Vigh T, Dravavolgyi G, Soti PL, Pataki H, Igricz T, Wagner I, et al. Predicting final product properties of melt extruded solid dispersions from process parameters using Raman spectrometry. J Pharm Biomed Anal. 2014;98:166–77. doi:10.1016/j.jpba.2014.05.025.
Zhang J, Ying Y, Pielecha-Safira B, Bilgili E, Ramachandran R, Romanach R, et al. Raman spectroscopy for in-line and off-line quantification of poorly soluble drugs in strip films. Int J Pharm. 2014;475(1-2):428–37. doi:10.1016/j.ijpharm.2014.08.051.
Gala U, Chauhan H. Principles and applications of Raman spectroscopy in pharmaceutical drug discovery and development. Expert Opin Drug Discov. 2014;1-20.
Barnes SE, Brown EC, Sibley MG, Edwards HGM, Scowen IJ, Coates PD. Vibrational spectroscopic and ultrasound analysis for in-process characterization of high-density polyethylene/polypropylene blends during melt extrusion. Appl Spectrosc. 2005;59(5):611–9.
Barnes SE, Brown EC, Sibley MG, Edwards HGM, Coates PD. Vibrational spectroscopic and ultrasound analysis for the in-process monitoring of poly(ethylene vinyl acetate) copolymer composition during melt extrusion. Analyst. 2005;130(3):286. doi:10.1039/b416244g.
Barnes SE, Sibley MG, Edwards HGM, Coates PD. Process monitoring of polymer melts using in-line spectroscopy. Trans Inst Measure Contrl. 2007;29(5):453–65.
Tumuluri VS, Kemper MS, Lewis IR, Prodduturi S, Majumdar S, Avery BA, et al. Off-line and on-line measurements of drug-loaded hot-melt extruded films using Raman spectroscopy. Int J Pharm. 2008;357(1-2):77–84. doi:10.1016/j.ijpharm.2008.01.036.
Saerens L, Adriaensens P, Carleer R, Vervaet C, Remon JP, De Beer T. Raman spectroscopy for the in-line polymer-drug quantification and solid state characterization during pharmaceutical hot-melt extrusion process. Pharm Sci. 2011.
Saerens L, Vervaet C, Remon JP, De Beer T. Visualization and process understanding of material behavior in the extrusion barrel during a hot-melt extrusion process using Raman spectroscopy. Anal Chem. 2013;85(11):5420–9. doi:10.1021/ac400097t.
Saerens L, Ghanam D, Raemdonck C, Francois K, Manz J, Kruger R, et al. In-line solid state prediction during pharmaceutical hot-melt extrusion in a 12 mm twin screw extruder using Raman spectroscopy. Eur J Pharm Biopharm. 2014;87(3):606–15. doi:10.1016/j.ejpb.2014.03.002.
Saerens L, Segher N, Vervaet C, Remon JP, De Beer T. Validation of an in-line Raman spectroscopic method for continuous active pharmaceutical ingredient quantification during pharmaceutical hot-melt extrusion. Anal Chim Acta. 2014;806:180–7. doi:10.1016/j.aca.2013.11.020.
Gilmor C, Balke ST, Calidonio F, Rom-Roginski A. In-line color monitoring of polymers during extrusion using a charge coupled device spectrometer. Polym Eng Sci. 2003;43(2):356–68.
Valadez-Blanco R, Virdi AIS, Balke ST, Diosady LL. In-line colour monitoring during food extrusion: sensitivity and correlation with product colour. Food Res Int. 2007;40(9):1129–39. doi:10.1016/j.foodres.2007.06.008.
Wang Y, Steinhoff B, Brinkmann C, Alig I. In-line monitoring of the thermal degradation of poly(l-lactic acid) during melt extrusion by UV–vis spectroscopy. Polymer. 2008;49(5):1257–65. doi:10.1016/j.polymer.2008.01.010.
Becker W, Guschin V, Mikonsaari I, Teipel U, Kolle S, Weiss P. Turbidimetric method for the determination of particle sizes in polypropylene/clay-composites during extrusion. Anal Bioanal Chem. 2016. doi:10.1007/s00216-016-0038-3.
Wallace VP, Taday PF, Fitzgerald AJ, Woodward RM, Cluff J, Pye RJ, et al. Terahertz pulsed imaging and spectroscopy for biomedical and pharmaceutical applications. Farad Discuss. 2004;126:255. doi:10.1039/b309357n.
Zeitler JA, Taday PF, Newnham DA, Pepper M, Gordon KC, Rades T. Terahertz pulsed spectroscopy and imaging in the pharmaceutical setting–a review. J Pharm Pharmacol. 2007;59(2):209–23. doi:10.1211/jpp.59.2.0008.
Shen YC. Terahertz pulsed spectroscopy and imaging for pharmaceutical applications: a review. Int J Pharm. 2011;417(1-2):48–60. doi:10.1016/j.ijpharm.2011.01.012.
Dohi M, Momose W, Yoshino H, Hara Y, Yamashita K, Hakomori T, et al. Application of terahertz pulse imaging as PAT tool for non-destructive evaluation of film-coated tablets under different manufacturing conditions. J Pharm Biomed Anal. 2016;119:104–13. doi:10.1016/j.jpba.2015.11.046.
Wu H, Heilweil EJ, Hussain AS, Khan MA. Process analytical technology (PAT): effects of instrumental and compositional variables on terahertz spectral data quality to characterize pharmaceutical materials and tablets. Int J Pharm. 2007;343(1-2):148–58. doi:10.1016/j.ijpharm.2007.05.014.
Cogdill RP, Forcht RN, Shen Y, Taday PF, Creekmore JR, Anderson CA, et al. Comparison of terahertz pulse imaging and near-infrared spectroscopy for rapid, non-destructive analysis of tablet coating thickness and uniformity. J Pharm Innov. 2007;2(1-2):29–36. doi:10.1007/s12247-007-9004-0.
Zhong S, Shen Y-C, Ho L, May RK, Zeitler JA, Evans M, et al. Non-destructive quantification of pharmaceutical tablet coatings using terahertz pulsed imaging and optical coherence tomography. Opt Laser Eng. 2011;49(3):361–5. doi:10.1016/j.optlaseng.2010.11.003.
Lin H, Dong Y, Shen Y, Zeitler JA. Quantifying pharmaceutical film coating with optical coherence tomography and terahertz pulsed imaging: an evaluation. J Pharm Sci. 2015;104(10):3377–85. doi:10.1002/jps.24535.
Chantry GW. Submillimeter spectroscopy a guide to the theoretical and experimental physics of the far infrared. Yew York: Acad. 1971;178:386.
Strachan CJ, Taday PF, Newnham DA, Gordon KC, Zeitler JA, Pepper M, et al. Using terahertz pulsed spectroscopy to quantify pharmaceutical polymorphism and crystallinity. J Pharm Sci. 2005;94(4):837–46. doi:10.1002/jps.20281.
Strachan CJ, Rades T, Newnham DA, Gordon KC, Pepper M, Taday PF. Using terahertz pulsed spectroscopy to study crystallinity of pharmaceutical materials. Chem Phys Lett. 2004;390(1-3):20–4. doi:10.1016/j.cplett.2004.03.117.
Zeitler JA, Newnham DA, Taday PF, Threlfall TL, Lancaster RW, Berg RW, et al. Characterization of temperature-induced phase transitions in five polymorphic forms of sulfathiazole by terahertz pulsed spectroscopy and differential scanning calorimetry. J Pharm Sci. 2006;95(11):2486–98. doi:10.1002/jps.20719.
Takeuchi I, Tomoda K, Nakajima T, Terada H, Kuroda H, Makino K. Estimation of crystallinity of trehalose dihydrate microspheres by usage of terahertz time-domain spectroscopy. J Pharm Sci. 2012;101(9):3465–72. doi:10.1002/jps.23147.
Takebe G, Kawada Y, Akiyama K, Takahashi H, Takamoto H, Hiramatsu M. Evaluation of drug crystallinity in aqueous suspension using terahertz time-domain attenuated total reflection spectroscopy. J Pharm Sci. 2013;102(11):4065–71. doi:10.1002/jps.23716.
Darkwah J, Smith G, Ermolina I, Mueller-Holtz M. A THz spectroscopy method for quantifying the degree of crystallinity in freeze-dried gelatin/amino acid mixtures: an application for the development of rapidly disintegrating tablets. Int J Pharm. 2013;455(1-2):357–64. doi:10.1016/j.ijpharm.2013.06.073.
Smith G, Hussain A, Bukhari NI, Ermolina I. Quantification of residual crystallinity of ball-milled, commercially available, anhydrous β-lactose by differential scanning calorimetry and terahertz spectroscopy. J Therm Anal Calorim. 2015;121(1):327–33. doi:10.1007/s10973-015-4469-4.
Krumbholz N, Hochrein T, Vieweg N, Hasek T, Kretschmer K, Bastian M, et al. Monitoring polymeric compounding processes inline with THz time-domain spectroscopy. Polym Test. 2009;28(1):30–5. doi:10.1016/j.polymertesting.2008.09.009.
Peters O, Schwerdtfeger M, Wietzke S, Sostmann S, Scheunemann R, Wilk R, et al. Terahertz spectroscopy for rubber production testing. Polym Test. 2013;32(5):932–6. doi:10.1016/j.polymertesting.2013.05.003.
Buckin V, O’Driscoll B, Smyth C, Alting AC, Visschers RW. Ultrasonic spectroscopy for material analysis. recent advances. Spectrosc Eur. 2003;15(1):20–5.
Stillhart C, Kuentz M. Comparison of high-resolution ultrasonic resonator technology and Raman spectroscopy as novel process analytical tools for drug quantification in self-emulsifying drug delivery systems. J Pharm Biomed Anal. 2012;59:29–37. doi:10.1016/j.jpba.2011.10.018.
Stelzer T, Pertig D, Ulrich J. Ultrasonic crystallization monitoring technique for simultaneous in-line measurement of liquid and solid phase. J Cryst Growth. 2013;362:71–6. doi:10.1016/j.jcrysgro.2011.11.027.
Shukla A, Prakash A, Rohani S. Online measurement of particle size distribution during crystallization using ultrasonic spectroscopy. Chem Eng Sci. 2010;65(10):3072–9. doi:10.1016/j.ces.2010.01.034.
Li M, Wilkinson D, Patchigolla K, Mougin P, Roberts KJ, Tweedie R. On-line crystallization process parameter measurements using ultrasonic attenuation spectroscopy. Cryst Growth Des. 2004;4(5):955–63.
Medendorp J, Buice RGJ, Lodder RA. Acoustic-resonance spectrometry as a process analytical technology for the quantification of active pharmaceutcial ingredient in semi-solids. AAPS PharmSciTech. 2006;7(3):1–8.
Kim H-J, Kim K-J. Quantitative study on polymorphic form in solution crystallization of clopidogrel hydrogen sulfate. Ind Eng Chem Res. 2009;48:11133–9.
Bonacucina G, Perinelli DR, Cespi M, Casettari L, Cossi R, Blasi P, et al. Acoustic spectroscopy: a powerful analytical method for the pharmaceutical field? Int J Pharm. 2016;503(1-2):174–95. doi:10.1016/j.ijpharm.2016.03.009.
Geers H, Witt W. Ultrasonic extinction for in-line measurement of particle size and concentration of suspensions and emulsions. Harrogate: Particulate System Analysis; 2003. p. 1–5.
Acknowledgements
This work was supported by AbbVie Deutschland GmbH and Co. KG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hitzer, P., Bäuerle, T., Drieschner, T. et al. Process analytical techniques for hot-melt extrusion and their application to amorphous solid dispersions. Anal Bioanal Chem 409, 4321–4333 (2017). https://doi.org/10.1007/s00216-017-0292-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0292-z