Abstract
Recombinase polymerase amplification (RPA) is an elegant method for the rapid, isothermal amplification of nucleic acids. Here, we elucidate the optimal surface chemistry for rapid and efficient solid-phase RPA, which was fine-tuned in order to obtain a maximum signal-to-noise ratio, defining the optimal DNA probe density, probe-to-lateral spacer ratio (1:0, 1:1, 1:10 and 1:100) and length of a vertical spacer of the probe as well as investigating the effect of different types of lateral spacers. The use of different labelling strategies was also examined in order to reduce the number of steps required for the analysis, using biotin or horseradish peroxidase-labelled reverse primers. Optimisation of the amplification temperature used and the use of surface blocking agents were also pursued. The combination of these changes facilitated a significantly more rapid amplification and detection protocol, with a lowered limit of detection (LOD) of 1 · 10−15 M. The optimised protocol was applied to the detection of Francisella tularensis in real samples from hares and a clear correlation with PCR and qPCR results observed and the solid-phase RPA demonstrated to be capable of detecting 500 fM target DNA in real samples.

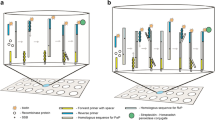
Relative size of thiolated lateral spacers tested versus the primer and the uvsx recombinase protein.




Similar content being viewed by others
References
Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLos Biol. 2006;7:1115–21.
Xu C, Li L, Jin W, Wan Y. Recombinase polymerase amplification (RPA) of CaMV-35S promoter and nos terminator for rapid detection of genetically modified crops. IJMS. 2014;10:18197–205.
Silva G, Bömer M, Nkere C, Lava Kumar P, Seal SE. Rapid and specific detection of Yam mosaic virus by reverse-transcription recombinase polymerase amplification. J Virol Methods. 2015; 138–144.
Boyle DS, McNerney R, Teng Low H, Leader BT, Pérez-Osorio AC, Meyer JC, et al. Rapid detection of Mycobacterium tuberculosis by recombinase polymerase amplification. PLoS ONE. 2014;8:e103091.
Abd El W, El-Deeb A, El-Tholoth M, Abd El Kader H, Ahmed A, Hassan S, et al. A portable reverse transcription recombinase polymerase amplification assay for rapid detection of foot-and-mouth disease virus. PLoS ONE. 2013;8:e71642.
Abd El Wahed M, Weidmann, Hufert FT. Diagnostics-in-a-Suitcase: Development of a portable and rapid assay for the detection of the emerging avian influenza A (H7N9) virus. J Clin Virol. 2015; 16–21.
Teoh B-T, Sam S-S, Tan K-K, Danlami MB, Shu M-H, Johari J, et al. Early detection of dengue virus by use of reverse transcription-recombinase polymerase amplification. J Clin Microbiol. 2015;3:830–7.
Crannell ZA, Castellanos-Gonzalez A, Irani A, Rohrman B, White AC, Richards-Kortum R. Nucleic acid test to diagnose Cryptosporidiosis: lab assessment in animal and patient specimens. Anal Chem. 2014;5:2565–71.
Crannell ZA, Cabada MM, Castellanos-Gonzalez A, Irani A, White AC, Richards-Kortum R. Recombinase polymerase amplification-based assay to diagnose Giardia in stool samples. Am J Trop Med. 2015;3:583–7.
Kersting S, Rausch V, Bier FF, von Nickisch-Rosenegk M. Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar J. 2014;13:99.
Tortajada-Genaro LA, Santiago-Felipe S, Amasia M, Russom A, Maquieira A. Isothermal solid-phase recombinase polymerase amplification on microfluidic digital versatile discs (DVDs). RSC Adv. 2015;38:29987–95.
Santiago-Felipe S, Tortajada-Genaro LA, Morais S, Puchades R, Maquieira Á. Isothermal DNA amplification strategies for duplex microorganism detection, Food Chem. 2015; 509–515.
Santiago-Felipe S, Tortajada-Genaro LA, Morais S, Puchades R, Maquieira Á. Hybridisation and detection by a disc-based method. Sensor Actuat B-Chem. 2014; 273–281.
del Río JS, Yehia Adly N, Acero-Sánchez JL, Henry OYF, O’Sullivan CK. Electrochemical detection of Francisella tularensis genomic DNA using solid-phase recombinase polymerase amplification. Biosens Bioelectron. 2014; 674–678.
Sakai K, Trabasso P, Moretti M, Mikami Y, Kamei K, Gonoi T. Identification of fungal pathogens by visible microarray system in combination with isothermal gene amplification. Mycopathologia. 2014;1–2:11–26.
Kersting S, Rausch V, Bier FF, von Nickisch-Rosenegk M. Multiplex isothermal solid-phase recombinase polymerase amplification for the specific and fast DNA-based detection of three bacterial pathogens. Mikrochim Acta. 2014;13–14:1715–23.
Shin Y, Perera AP, Kim KW, Park MK. Real-time, label-free isothermal solid-phase amplification/detection (ISAD) device for rapid detection of genetic alteration in cancers. Lab Chip. 2013;11:2106–14.
Sabaté del Río J, Steylaerts T, Henry OYF, Bienstman P, Stakenborg T, Van Roy W, et al. Real-time and label-free ring-resonator monitoring of solid-phase recombinase polymerase amplification. Biosens Bioelectron. 2015;73:130–7.
Shin Y, Perera AP, Tang WY, Fu DL, Liu Q, Sheng JK, et al. A rapid amplification/detection assay for analysis of Mycobacterium tuberculosis using an isothermal and silicon bio-photonic sensor complex. Biosens Bioelectron. 2015;68:390–6.
Sjöstedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann NY Acad Sci. 2007;1105:1–29.
Euler M, Wang Y, Otto P, Tomaso H, Escudero R, Anda P, et al. Recombinase polymerase amplification assay for rapid detection of Francisella tularensis. J Clin Microbiol. 2012;7:2234–8.
Sabaté del Rio J. P. Conejeras and CK O’ Sullivan, Electrochemical detection of Piscirickettsia salmonis genomic DNA from salmon samples using solid-phase recombinase polymerase amplification. Anal Bioanal Chem. 2016;408:8611–20.
Acknowledgements
This work has been carried out with partial financial support from Spanish Ministerio de Economía y Competitividad (SEASENSING BIO2014-56024-C2-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jonathan Sabaté del Río, Ivan Magriñà Lobato and Olena Mayboroda contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 470 kb)
Rights and permissions
About this article
Cite this article
del Río, J.S., Lobato, I.M., Mayboroda, O. et al. Enhanced solid-phase recombinase polymerase amplification and electrochemical detection. Anal Bioanal Chem 409, 3261–3269 (2017). https://doi.org/10.1007/s00216-017-0269-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0269-y