Abstract
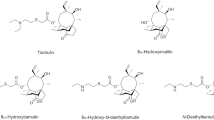
For the first time, a sensitive and specific method was developed and fully validated for the quantification of the EU marker residue of tiamulin, 8-α-hydroxy-mutilin, in rabbit muscle and liver tissues using liquid chromatography combined with positive heated electrospray ionization triple quadrupole mass spectrometry. The mass spectrometer was operated in the selected reaction monitoring (SRM) mode with selection of the [M + H]+ ion in both quadrupoles 1 and 3, resulting in the SRM transition m/z 337.25 > 337.25 for quantification. Chromatography was performed using a Hypersil Gold C18 column using a gradient elution program with water and methanol as mobile phases. The sample preparation procedure for the analysis of 8-α-hydroxy-mutilin in liver and muscle samples consisted of three main steps: (1) extraction of the tissue matrix using 0.1 N hydrochloric acid/acetone (50/50, v/v), (2) hydrolysis of tiamulin and metabolites to 8-α-hydroxy-mutilin in alkaline medium at 45 °C, and (3) liquid–liquid extraction in acidic medium using ethyl acetate. This is the first method presenting fully validated results, encompassing a linearity of 50 to 2,000 μg/kg, within-run and between-run accuracy and precision, limit of quantification (50 μg/kg for both muscle and liver tissues), limit of detection (muscle, 11.9 μg/kg; liver, 20.6 μg/kg), extraction recovery (muscle, 66.2 %; liver, 75.5 %), signal suppression and enhancement (muscle, 51.7 %; liver, 43.3 %), carryover, applicability and practicability, and stability during storage and analysis. This novel method is therefore sensitive enough to be used for residue depletion studies of tiamulin in rabbits and for food safety monitoring with respect to MRL compliance of residues.



Similar content being viewed by others
References
Lykkeberg AK, Cornett C, Halling-Sorensen B, Hansen SH (2006) Isolation and structural elucidation of tiamulin metabolites formed in liver microsomes of pigs. J Pharm Biomed Anal 42(2):223–231
European Commission (2000) Committee for Veterinary Medicinal Products. Tiamulin, summary report (2)—extension to rabbits, EMEA/MRL/724/00-Final
Pan X, Qiang ZM, Ben WW, Chen MX (2011) Simultaneous determination of three classes of antibiotics in the suspended solids of swine wastewater by ultrasonic extraction, solid-phase extraction and liquid chromatography-mass spectrometry. J Environ Sci-China 23(10):1729–1737
Schlusener MP, Bester K, Spiteller M (2003) Determination of antibiotics such as macrolides, ionophores and tiamulin in liquid manure by HPLC-MS/MS. Anal Bioanal Chem 375(7):942–947
Cheng LL, Zhang SX, Shen JZ, Wang ZH, Li YS, Feng PS (2009) A rapid high performance liquid chromatographic method for determination of tiamulin residue in swine tissues. Chin J Anal Chem 37(5):718–720
Chen HC, Cheng SH, Tsai YH, Hwang DF (2006) Determination of tiamulin residue in pork and chicken by solid phase extraction and HPLC. J Food Drug Anal 14(1):80–83
Nozal MJ, Bernal JL, Martin MT, Jimenez JJ, Bernal J, Higes M (2006) Trace analysis of tiamulin in honey by liquid chromatography-diode array-electrospray ionization mass spectrometry detection. J Chromatogr A 1116(1–2):102–108
Markus JR, Sherma J (1993) Method. V. Gas chromatographic/mass spectrometric confirmation of 8-hydroxymutilin, a tiamulin metabolite, in swine liver extracts. J AOAC Int 76(2):459–460
Markus JR, Sherma J (1993) Method. IV. Gas chromatographic determination of tiamulin residues in swine liver. J AOAC Int 76(2):451–458
Burton D, Airs D, Brewin S, Todd M (2006) The analysis of tiamulin and 8α-hydroxy mutilin in various animal tissues and fluids by LC-MS. J Vet Pharm Therap 29(S1):160–161
European Union (2005) Volume 8, notice to applicants, veterinary medicinal products: establishment of maximum residue limits (MRLs) for residues of veterinary products in foodstuffs of animal origin: development and validation of a proposed regulatory method, EMEA/CVMP/573/00.
VICH (2012) VICH GL49, guidance for industry, studies to evaluate the metabolism and residue kinetics of veterinary drugs in food producing animals: validation of analytical methods used in residue depletion studies.
Knecht J, Stork G (1974) Percentage and logarithmic procedures for calculation of calibration curves. Fresen Z Anal Chem 270(2):97–99
Acknowledgments
The technical assistance of Jelle Lambrecht is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection on Hormone and Veterinary Drug Residue Analysis with guest editors Siska Croubels, Els Daeseleire, Sarah De Saeger, Peter Van Eenoo, and Lynn Vanhaecke.
Siegrid De Baere and Mathias Devreese shared first authorship.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 40 kb)
Rights and permissions
About this article
Cite this article
De Baere, S., Devreese, M., Maes, A. et al. Quantification of 8-α-hydroxy-mutilin as marker residue for tiamulin in rabbit tissues by high-performance liquid chromatography-mass spectrometry. Anal Bioanal Chem 407, 4437–4445 (2015). https://doi.org/10.1007/s00216-014-8437-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8437-9