Abstract
One novel tissue engineering approach to mimic in vivo bone formation is the use of aggregate or micromass cultures. Various qualitative and quantitative techniques, such as histochemical staining, protein assay kits and RT-PCR, have been used previously on cellular aggregate studies to investigate how these intricate arrangements lead to mature bone tissue. However, these techniques struggle to reveal spatial and temporal distribution of proliferation and mineralization simultaneously. Synchrotron-based Fourier transform infrared microspectroscopy (micro-FTIR) offers a unique insight at the molecular scale by coupling high IR sensitivity to organic matter with the high spatial resolution allowed by diffraction limited SR microbeam. This study is set to investigate the effects of culture duration and aggregate size on the dynamics and spatial distribution of calcification in engineered bone aggregates by a combination of micro-FTIR and scanning electron microscopy (SEM)/energy-dispersive X-ray spectroscopy (EDX). A murine bone cell line has been used, and small/large bone aggregates have been induced using different chemically treated culture substrates. Our findings suggest that bone cell aggregate culturing can greatly increase levels of mineralization over short culture periods. The size of the aggregates influences mineralisation rates with larger aggregates mineralizing at a faster rate than their smaller counterparts. The micro-FTIR mapping has demonstrated that mineralization in the larger aggregates initiated from the periphery and spread to the centre, whilst the smaller aggregates have more minerals in the centre at the early stage and deposited more in the periphery after further culturing, implying that aggregate size influences calcification distribution and development over time. SEM/EDX data correlates well with the micro-FTIR results for the total mineral content. Thus, synchrotron-based micro-FTIR can accurately track mineralization process/mechanism in the engineered bone.

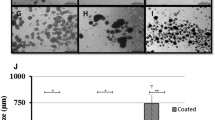
FTIR mapping images of PO4 regions showing the big intensity and distribution difference between small and large aggregates cultured for 72 h.





Similar content being viewed by others
References
Amini AR, Laurencin CT, Nukavarapu SP (2012) Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40(5):363–408
Meinel L et al (2004) Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng 32(1):112–22
Moore WR, Graves SE, Bain GI (2001) Synthetic bone graft substitutes. ANZ J Surg 71(6):354–61
Peres JA, Lamano T (2001) Strategies for stimulation of new bone formation: a critical review. Braz Dent J 22(6):443–8
Rossi MID et al (2005) Multicellular spheroids of bone marrow stromal cells: a three-dimensional in vitro culture system for the study of hematopoietic cell migration. Braz J Med Biol Res 38(10):1455–62
Aubin JE, Liu F, Malaval L, Gupta AK (1995) Osteoblast and chondroblast differentiation. Bone 17(2, Supplement 1):S77–83
Berrier AL, Yamada KM (2007) Cell–matrix adhesion. J Cell Physiol 213(3):565–73
Schecroun N, Delloye C (2003) Bone-like nodules formed by human bone marrow stromal cells: comparative study and characterization. Bone 32(3):252–60
Ma D et al (2011) Engineering Injectable bone using bone marrow stromal cell aggregates. Stem Cells Dev 20(6):989–99
Dong P et al (2014) 3D osteocyte lacunar morphometric properties and distributions in human femoral cortical bone using synchrotron radiation micro-CT images. Bone 60:172–85
Petibois C, Piccinini M, Guidi MC, Marcelli A (2010) Facing the challenge of biosample imaging by FTIR with a synchrotron radiation source. J Synchrotron Radiat 17(1):1–11
Miller LM, Dumas P (2006) Chemical imaging of biological tissue with synchrotron infrared light. Biochim Biophys Acta 1758(7):846–57
Paschalis EP, Mendelsohn R, Boskey AL (2011) Infrared assessment of bone quality: a review. Clin Orthop 469(8):2170–8
Miller LM, Little W, Schirmer A, Sheik F, Busa B, Judex S (2007) Accretion of bone quantity and quality in the developing mouse skeleton. J Bone Miner Res 22(7):1037–45
Practical Guide to Infrared Microspectroscopy [Internet]. [cited 2014 Jun 26]. Available from: http://www.crcpress.com/product/isbn/9780824794491
Carr GL, Williams GP (1997) Infrared microspectroscopy with synchrotron radiation. Proc. SPIE 3153, Accelerator-Based Infrared Sources and Applications, 51; doi:10.1117/12.290262
Wehbe K et al (2013) Investigation of blood vessels in glioblastoma at a micrometric scale: a comparative study by synchrotron and conventional micro-FTIR. Anal Methods 5(24):6925–32
Duncan WD, Williams GP (1983) Infrared synchrotron radiation from electron storage rings. Appl Opt 22(18):2914–23
Carr GL, Reffner JA, Williams GP (1995) Performance of an infrared microspectrometer at the NSLS. Rev Sci Instrum 66(2):1490
Reffner JA, Martoglio PA, Williams GP (1995) Fourier transform infrared microscopical analysis with synchrotron radiation: the microscope optics and system performance (invited). Rev Sci Instrum 66(2):1298–302
Marcelli A, Cinque G (2011) Infrared synchrotron radiation beamlines: high brilliance tools for IR spectromicroscopy. In biomedical applications of synchrotron infrared microspectroscopy: a practical approach. Marcelli A and Cinque G eds, RSC, UK
Kato Y, Boskey A, Spevak L, Dallas M, Hori M, Bonewald LF (2001) Establishment of an osteoid preosteocyte-like Cell MLO-A5 that spontaneously mineralizes in culture. J Bone Miner Res 16(9):1622–33
Aydin HM, Hu B, Sulé Suso J, El Haj A, Yang Y (2011) Study of tissue engineered bone nodules by Fourier transform infrared spectroscopy. Analyst 136(4):775
Carson FL, Hladik C (2009) Histotechnology, American Society of Clinical Pathologists Press, Chicago
Faibish D, Gomes A, Boivin G, Binderman I, Boskey A (2005) Infrared imaging of calcified tissue in bone biopsies from adults with osteomalacia. Bone 36(1):6–12
Gadaleta SJ, Camacho NP, Mendelsohn R, Boskey AL (1996) Fourier transform infrared microscopy of calcified turkey leg tendon. Calcif Tissue Int 58(1):17–23
Deegan AJ, Aydin HM, Hu B, Konduru S, Kuiper JH, Yang Y (2014) A facile in vitro model to study rapid mineralization in bone tissues. Biomed Eng OnLine 13(1):136
Kennedy DF (1991) Studies of peptides forming 310-and. alpha.-helixes and. beta.-bend ribbon structures in organic solution and in model biomembranes by Fourier transform infrared spectroscopy. Biochemistry (Mosc) 30(26):6541
Nancollas GH et al (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38(5):617–27
Acknowledgments
The authors are grateful for the financial support from NHS Orthopaedic Charity funding (001502) and Diamond light source for beamtime on B22 (Diamond Light Source fund SM8637).
Conflict of interest
There is no conflict of interest of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deegan, A.J., Cinque, G., Wehbe, K. et al. Tracking calcification in tissue-engineered bone using synchrotron micro-FTIR and SEM. Anal Bioanal Chem 407, 1097–1105 (2015). https://doi.org/10.1007/s00216-014-8316-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8316-4