Abstract
Polymers capable of dynamic bonding/debonding reactions are of great interest in modern day research. Potential applications can be found in the fields of self-healing materials or printable networks. Since temperature is often used as a stimulus for triggering reversible bonding reactions, an analysis operating at elevated temperatures is very useful for the in situ investigation of the reaction mechanism, as unwanted side effects can be minimized when performing the analyses at the same temperature at which the reactions occur. A temperature-dependent size exclusion chromatographic system (TD SEC) has been optimized for investigating the kinetics of retro Diels−Alder-based depolymerization of Diels−Alder polymers. The changing molecular weight distribution of the analyzed polymers during depolymerization gives valuable quantitative information on the kinetics of the reactions. Adequate data interpretation methods were developed for the correct evaluation of the chromatograms. The results are confirmed by high-temperature dynamic light scattering, thermogravimetric analysis, and time-resolved nuclear magnetic resonance spectroscopy at high temperatures. In addition, the SEC system and column material stability under application conditions were assessed using thermoanalysis methods, infrared spectroscopy, nitrogen physisorption, and scanning electron microscopy. The findings demonstrate that the system is stable and, thus, we can reliably characterize such dynamically bonding/debonding systems with TD SEC.

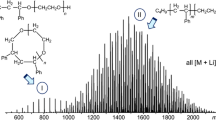
3D illustration of chromatograms of a polymer after different times of a depolymerization reaction








Similar content being viewed by others
References
Haining L, Yuan X, Zaisheng C, Jie S (2011) Microporous membrane with temperature-sensitive breathability based on PU/PNIPAAm semi-IPN. J Appl Polym Sci 124:2–8
Zhang S, Yu F (2011) Piezoelectric materials for high temperature sensors. J Am Ceram Soc 94:3153–3170
Islam A, Yasin T, Bano I, Riaz M (2011) Controlled release of aspirin from pH-sensitive chitosan/poly(vinyl alcohol) hydrogel. J Appl Polym Sci 124:4184–92
Kataoka K, Harada A, Nagasaki Y (2001) Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–131
Wang CH, Sidhu K, Yang T, Zhang J, Shanks R (2012) Interlayer self-healing and toughening of carbon fibre/epoxy composites using copolymer films. Compos Appl Sci Manuf 43:512–518
Maeda T, Otsuka H, Takahara A (2009) Dynamic covalent polymers: reorganizable polymers with dynamic covalent bonds. Prog Poly Sci 34:581–604
Trask RS, Williams HR, Bond IP (2007) Self-healing polymer composites: mimicking nature to enhance performance. Bioinspir Biomim 2:1–9
Guimard NK, Oehlenschlaeger KK, Zhou J, Hilf S, Schmidt FG, Barner-Kowollik C (2012) Current trends in the field of self-healing materials. Macromol Chem Phys 213:131–143
Kwart H, King K (1968) The reverse Diels–Alder or retrodiene reaction. Chem Rev 68:415–439
Zhou J, Guimard NK, Inglis AJ, Namazian M, Lin CY, Coote ML, Spyrou E, Hilf S, Schmidt FG, Barner-Kowollik C (2012) Thermally reversible Diels–Alder-based polymerization: an experimental and theoretical assessment. Pol Chem 3:628–639
Sanyal A (2010) Diels–Alder cycloaddition-cycloreversion: a powerful combo in materials design. Macromol Chem Phys 211:1417–1425
Park JS, Darlington T, Starr AF, Takahashi K, Riendeau J, Thomas Hahn H (2010) Multiple healing effect of thermally activated self-healing composites based on Diels–Alder reaction. Compos Sci Technol 70:2154–2159
Froimowicz P, Frey H, Landfester K (2011) Towards the generation of self-healing materials by means of a reversible photo-induced approach. Macromol Rapid Commun 32:468–473
Chung C, Roh Y, Cho S, Kim J (2004) Crack healing in polymeric materials via photochemical [2+2] cycloaddition. Chem Mater 16:3982–3984
Pasch H (2001) Recent developments in polyolefin characterization. Macromol Sy 165:91–98
Cho H, Park S, Ree M, Chang T, Jung JC, Zin WC (2006) High temperature size exclusion chromatography. Macromol Res 14:383–386
Ginzburg A, Macko T, Dolle V, Brüll R (2011) Characterization of polyolefins by comprehensive high-temperature two-dimensional liquid chromatography (HT 2D-LC). Europ Polym J 47:319–329
Park S, Cho H, Kim Y, Ahn S, Chang T (2007) Fast size-exclusion chromatography at high temperature. J Chrom A 1157:96–100
Guimard NK, Ho J, Brandt J, Lin Y, Namazian M, Jan O, Oehlenschlaeger KK, Hilf S, Lederer A, Schmidt FG, Coote ML, Barner-Kowollik C (2013) Harnessing entropy to direct the bonding/debonding of polymer systems based on reversible chemistry. Chem Sci 4:2752–2759
Li Y, Fan Y, Ma J (2001) The thermal properties of porous polydivinylbenzene beads. React Funct Polym 50:57–65
Straus S, Madorsky SL (1961) Thermal stability of polydivinylbenzene and of copolymers of styrene with divinylbenzene and with trivinylbenzene. J Res Nat Bur Stand A Phys Chem 65A:243–248
De Santa MLC, Souza MAV, Santos FR, Rubenich LMS, Ferreira MDJF, Sá RMP (2008) Thermogravimetric and spectrometric characterizations of poly(styrene-co-divinylbenzene) containing phosphinic and phosphonic acid groups. Polym Eng Sci 48:1897–1900
Grubisic Z, Rempp P, Benoit H (1967) A universal calibration for gel permeation chromatography. Polym Lett 5:753–759
Socrates G (1980) Infrared characteristic group frequencies. Wiley, Chichester
Grimes BA, Skudas R, Unger KK, Lubda D (2007) Pore structural characterization of monolithic silica columns by inverse size-exclusion chromatography. J Chrom A 1144:14–29
Yao Y, Lenhoff AM (2004) Determination of pore size distributions of porous chromatographic adsorbents by inverse size-exclusion chromatography. J Chrom A 1037:273–282
DePhillips P, Lenhoff AM (2000) Pore size distributions of cation-exchange adsorbents determined by inverse size-exclusion chromatography. J Chrom A 883:39–54
Potschka M (1987) Universal calibration of gel permeation chromatography and determination of molecular shape in solution. Anal Biochem 162:47–64
Renn CN, Synovec RE (1992) Effect of temperature on separation efficiency for high-speed size exclusion chromatography. Anal Chem 64:479–484
Antia FD, Horvath C (1988) High-performance liquid chromatography at elevated temperatures: examination of conditions for the rapid separation of large molecules. J Chrom 435:1–15
Greibrokk T, Andersen T (2003) High-temperature liquid chromatography. J Chrom A 1000:743–755
AgilentTechnologies (2013) Organic GPC/SEC columns product guide
Peterson JD, Vyazovkin S, Wight C a (2001) Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly(propylene). Macromol Chem Phys 202:775–784
Borchard W (1978) Thermodynamic reflections on the course of polymer swelling curves. Europ Polym J 14:661–664
Cantow MJR, Porter RS, Julian F (1967) Effect of temperature and polymer type on gel permeation chromatography. J Polym Sci A 15:987–991
Oehlenschlaeger KK, Guimard NK, Brandt J, Mueller JO, Lin CY, Hilf S, Lederer A, Coote ML, Schmidt FG, Barner-Kowollik C (2013) Fast and catalyst-free hetero-Diels–Alder chemistry for on demand cyclable bonding/debonding materials. Polym Chem. doi:10.1039/c3py00476g
Acknowledgments
A.L., J.B., N. G., and C.B.-K. thank Evonik Industries AG for continued financial support and the excellent collaboration. Kerstin Arnhold is acknowledged for help with the thermoanalysis, and Mikhail Malanin for the IR analysis and interpretation, while Viktoria Albrecht is thanked for nitrogen physisorption studies and Hartmut Komber for the NMR measurements. C.B.-K. is grateful for continued support from the Karlsruhe Institute of Technology and the Helmholtz association in its Science and Technology of Nanosystems and BioInterfaces programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Separation and Characterization of Natural and Synthetic Macromolecules with guest editors Albena Lederer and Peter J. Schoenmakers.
Rights and permissions
About this article
Cite this article
Brandt, J., Guimard, N.K., Barner-Kowollik, C. et al. Temperature-dependent size exclusion chromatography for the in situ investigation of dynamic bonding/debonding reactions. Anal Bioanal Chem 405, 8981–8993 (2013). https://doi.org/10.1007/s00216-013-7203-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7203-8