Abstract
There is an increasing demand for easy and cost-effective methods to screen the toxicological impact of the growing number of chemical mixtures being generated by industry. Such a screening method has been developed using viable, genetically modified green fluorescent protein (GFP) reporter yeast that was magnetically functionalised and held within a microfluidic device. The GFP reporter yeast was used to detect genotoxicity by monitoring the exposure of the cells to a well-known genotoxic chemical (methyl methane sulfonate, MMS). The cells were magnetised using biocompatible positively charged PAH-stabilised magnetic nanoparticles with diameters around 15 nm. Gradient mixing was utilised to simultaneously expose yeast to a range of concentrations of toxins, and the effective fluorescence emitted from the produced GFP was measured. The magnetically enhanced retention of the yeast cells, with their facile subsequent removal and reloading, allowed for very convenient and rapid toxicity screening of a wide range of chemicals. This is the first report showing magnetic yeast within microfluidic devices in a simple bioassay, with potential applications to other types of fluorescent reporter yeast in toxicological and biomedical research. The microfluidic chip offers a simple and low-cost screening test that can be automated to allow multiple uses (adapted to different cell types) of the device on a wide range of chemicals and concentrations.

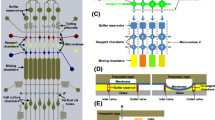
Multi-concentration chemical toxicity screening within a microsystem using a fluorescent reporter yeast, with the fluorescence increasing as the concentration of the genotoxic compound increases.


Similar content being viewed by others
References
European Commission Regulation (2006) Regulation (EC) No 882/2004 of the European parliament and of the council as regards community reference laboratories. Official J Eur Union L136:3–8
de Souza PR, Geibel J (1999) Direct observation of oxidative stress on the cell wall of Saccharomyces cerevisiae strains with atomic force microscopy. Mol Cell Biochem 201:17–24
Cahill PA, Knight AW, Billinton N, Barker MG, Walsh L, Keenan PO, Williams CV, Tweats DJ, Walmsley RM (2004) The GreenScreen genotoxicity assay: a screening validation programme. Mutagenesis 19:105–119
Michelini E, Leskinen P, Virta M, Kart M, Roda A (2005) A new recombinant cell-based bioluminescent assay for sensitive androgen-like compound detection. Biosens Bioelectron 20:2261–2267
VanGompel JV, Woestenborghs F, Beerens D, Mackie C, Cahill PA, Knight AW, Billington N, Tweats DJ, Walmsley RM (2005) An assessment of the utility of the yeast GreenScreen assay in pharmaceutical screening. Mutagenesis 20:449–454
Knight AW, Keenan PO, Goddard NJ, Fielden PR, Walmsley RM (2004) A yeast-based cytotoxicity and genotoxicity assay for environmental monitoring using novel portable instrumentation. J Environ Monitor 6:71–79
MacDonald MP, Spalding GC, Dholakia K (2003) Microfluidic sorting in an optical lattice. Nature 426:421–424
Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S (2003) Passively driven integrated microfluidic system for separation of motile sperm. Anal Chem 75:1671–1675
Rhee SW, Taylor AM, Tu CH, Cribbs DH, Cotman CW, Jeon NL (2004) Patterned cell culture inside microfluidic devices. Lab Chip 5:102–107
Zeringue HC, Rutledge JJ, Beebe DJ (2004) Early mammalian embryo development depends on cumulus removal technique. Lab Chip 5:86–90
Tourovskaia A, Figueroa-Masot X, Folch A (2004) Differentiation-on-a-chip: a microfluidic platform for long-term cell culture studies. Lab Chip 5:14–19
Verdich AA, Lima EA, Ivanov B, Ges I, Anderson ME, Wikswo JP, Baudenbacher FJ (2004) A microfluidic device to confine a single cardiac myocyte in a sub-nanoliter volume on planar microelectrodes for extracellular potential recordings. Lab Chip 4:357–362
Hung PJ, Lee PJ, Sabounchi P, Lin R, Lee LP (2005) Continuous perfusion microfluidic cell culture array for high-throughput cell-based assays. Biotechnol Bioeng 89:1–8
García-Alonso J, Greenway G, Hardege J, Haswell S (2009) A prototype microfluidic chip using fluorescent yeast for detection of toxic compounds. Biosens Bioelectron 24:1508–1511
Ye N, Qin J, Shi W, Liu X, Lin B (2007) Cell-based high content screening using an integrated microfluidic device. Lab Chip 7:1696–1705
García-Alonso J, Fakhrullin RF, Paunov VN (2010) Rapid and direct magnetization of GFP-reporter yeast for micro-screening systems. Biosens Bioelectron 25:1816–1819
Pamme N (2006) Magnetism and microfluidics. Lab Chip 6(1):24–38
Breeuwer P, Drocourt J-L, Bunschoten N, Zwietering MC, Rombouts FM, Abee T (1995) Characterization of uptake and hydrolysis of fluorescein diacetate and carboxyfluorescein diacetate by intracellular esterases in Saccharomyces cerevisiae, which result in accumulation of fluorescent product. Appl Environ Microbiol 61:1614–1619
Acknowledgements
We thank Dr. Andrew Knight Gentronix® for kindly supplying the yeast cells, Mr. Stephen Clark for technical support and Mrs. A. Lowry for the technical assistance with TEM. This work was funded by the European Commission, TESS COLL-CT-2006 project. The support by the Government of the Republic of Tatarstan (Algarish Grantlar Programmasi) is gratefully acknowledged by R.F.F.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Microorganisms for Analysis with Guest Editor Gérald Thouand
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 588 kb)
Rights and permissions
About this article
Cite this article
García-Alonso, J., Fakhrullin, R.F., Paunov, V.N. et al. Microscreening toxicity system based on living magnetic yeast and gradient chips. Anal Bioanal Chem 400, 1009–1013 (2011). https://doi.org/10.1007/s00216-010-4241-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-4241-3