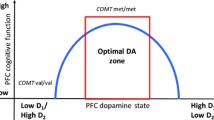
Abstract
Rationale
The catechol-O-methyl transferase (COMT) enzyme has been implicated in determining dopaminergic tone and the effects of delta-9-tetrahydrocannabinol (THC) in the human brain.
Objective
This study was designed to evaluate the effect of (1) a functional polymorphism and (2) acute pharmacological inhibition of COMT on the acute response to THC in humans.
Methods
Sub-study I: The effect of intravenous (IV) THC (0.05 mg/kg) was investigated in 74 healthy subjects genotyped for the COMT rs4680 (Val/Met) polymorphism in a 2-test-day double-blind, randomized, placebo-controlled study. Sub-study II: COMT rs4680 homozygous subjects (Val/Val and Met/Met) from sub-study I received the COMT enzyme inhibitor tolcapone (200 mg) followed by IV THC or placebo on two additional test days. Subjective, behavioral, and cognitive data were obtained periodically on each test day.
Results
Sub-study I: Val/Val individuals were most sensitive to THC-induced attention and working memory deficits. In contrast, the psychotomimetic and subjective effects of THC were not influenced by COMT genotype. Sub-study II: Tolcapone reduced THC-induced working memory deficits, but not THC’s psychotomimetic effects. Tolcapone and COMT genotype (met/met) were associated with an increased report of feeling “mellow.”
Conclusions
The interaction between COMT rs4680 polymorphisms and tolcapone on the cognitive, but not on the psychotomimetic and overall subjective effects of THC, suggests that modulation of dopaminergic signaling may selectively influence specific cannabinoid effects in healthy individuals. The role of dopaminergic signaling in the cognitive effects of cannabinoids should be considered in drug development efforts targeting these effects.
Clinicaltrials.gov registration
https://clinicaltrials.gov/ct2/show/NCT00678730?term=NCT00678730&rank=1
ClinicalTrials.gov Identifier: NCT00678730.




Similar content being viewed by others
References
Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF (2007) Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32:1011–1020
Barkus E, Morrison PD, Vuletic D, Dickson JC, Ell PJ, Pilowsky LS, Brenneisen R, Holt DW, Powell J, Kapur S (2011) Does intravenous Δ9-tetrahydrocannabinol increase dopamine release? A SPET study. J Psychopharmacol 25:1462–1468
Beaulieu J-M, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217
Behan ÁT, Hryniewiecka M, O'tuathaigh CM, Kinsella A, Cannon M, Karayiorgou M, Gogos JA, Waddington JL, Cotter DR (2012) Chronic adolescent exposure to delta-9-tetrahydrocannabinol in COMT mutant mice: impact on indices of dopaminergic, endocannabinoid and GABAergic pathways. Neuropsychopharmacology 37:1773–1783
Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD (2014) Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry 75:470–478
Bloomfield MA, Ashok AH, Volkow ND, Howes OD (2016) The effects of delta(9)-tetrahydrocannabinol on the dopamine system. Nature 539:369–377
Bossong MG, Van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, Van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS (2009) [Delta] 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 34:759–766
Bossong MG, Mehta MA, van Berckel BN, Howes OD, Kahn RS, Stokes PR (2015) Further human evidence for striatal dopamine release induced by administration of 9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology 232:2723–2729
Bossong MG, van Hell HH, Schubart CD, van Saane W, Iseger TA, Jager G, van Osch MJ, Jansma JM, Kahn RS, Boks MP (2019) Acute effects of ∆ 9-tetrahydrocannabinol (THC) on resting state brain function and their modulation by COMT genotype. Eur Neuropsychopharmacol
Brunner E, Domhof S, Langer F (2002) Nonparametric analysis of longitudinal data in factorial designs. Wiley, New York
Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A (2005) Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 57:1117–1127
Ceravolo R, Piccini P, Bailey DL, Jorga KM, Bryson H, Brooks DJ (2002) 18F-dopa PET evidence that tolcapone acts as a central COMT inhibitor in Parkinson’s disease. Synapse 43:201–207
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR (2004) Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75:807–821
Cohen K, Weinstein A (2018) The effects of cannabinoids on executive functions: evidence from cannabis and synthetic cannabinoids—a systematic review. Brain Sci 8:40
Cosker E, Schwitzer T, Ramoz N, Ligier F, Lalanne L, Gorwood P, Schwan R, Laprévote V (2017) The effect of interactions between genetics and cannabis use on neurocognition. A review. Prog Neuro-Psychopharmacol Biol Psychiatry
D’Souza DC, Braley G, Blaise R, Vendetti M, Oliver S, Pittman B, Ranganathan M, Bhakta S, Zimolo Z, Cooper T (2008) Effects of haloperidol on the behavioral, subjective, cognitive, motor, and neuroendocrine effects of Δ-9-tetrahydrocannabinol in humans. Psychopharmacology 198:587–603
Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A, Sirianni M, La Cascia C, Stilo SA, Marques TR (2012) Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry 72:811–816
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci 98:6917–6922
Giakoumaki SG, Roussos P, Bitsios P (2008) Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology 33:3058–3068
Goldman D, Weinberger DR, Malhotra AK, Goldberg TE (2009) The role of COMT Val158Met in cognition. Biol Psychiatry 65:e1–e2
Heinz A, Smolka MN (2006) The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev Neurosci 17:359–368
Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen H-U, Van Os J (2004) Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Bmj 330:11
Henquet C, Murray R, Linszen D, van Os J (2005) The environment and schizophrenia: the role of cannabis use. Schizophr Bull 31:608–612
Henquet C, Rosa A, Krabbendam L, Papiol S, Fananas L, Drukker M, Ramaekers JG, van Os J (2006) An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31:2748–2757
Henquet C, Rosa A, Delespaul P, Papiol S, Fananas L, van Os J, Myin-Germeys I (2009) COMT ValMet moderation of cannabis-induced psychosis: a momentary assessment study of 'switching on' hallucinations in the flow of daily life. Acta Psychiatr Scand 119:156–160
Hirvonen M, Nagren K, Rinne J, Pesonen U, Vahlberg T (2009) COMT Val158Met genotype does not alter cortical or striatal dopamine D2 receptor. Availability in vivo Mol Imaging Biol
Htun NC, Miyaki K, Zhao C, Muramatsu M, Sato N (2014) Epistasis effects of COMT and MTHFR on inter-individual differences in mental health: under the inverted U-shaped prefrontal dopamine model. Biochem Biophys Res Commun 451:574–579
Jorga KM (1998) Pharmacokinetics, pharmacodynamics, and tolerability of tolcapone: a review of early studies in volunteers. Neurology 50:S31–S38
Kantrowitz JT, Nolan KA, Sen S, Simen AA, Lachman HM, Bowers MB (2009) Adolescent cannabis use, psychosis and catechol-O-methyltransferase genotype in African Americans and Caucasians. Psychiatry Q 80:213–218
Lazenka MF, Tomarchio AJ, Lichtman AH, Greengard P, Flajolet M, Selley DE, Sim-Selley LJ (2015) Role of dopamine type 1 receptors and dopamine-and cAMP-regulated phosphoprotein Mr 32 kDa in Δ9-tetrahydrocannabinol–mediated induction of ΔFosB in the mouse forebrain. J Pharmacol Exp Ther 354:316–327
Morgan CJ, Freeman TP, Powell J, Curran HV (2016) AKT1 genotype moderates the acute psychotomimetic effects of naturalistically smoked cannabis in young cannabis smokers. Transl Psychiatry 6:e738
Munafò MR, Smith GD (2018) Robust research needs many lines of evidence. Nature Publishing Group
Muñoz-Arenas G, Paz-Bermúdez F, Báez-Cordero A, Caballero-Florán R, González-Hernández B, Florán B, Daniel Limón I (2015) Cannabinoid CB1 receptors activation and coactivation with D2 receptors modulate GABAergic neurotransmission in the globus pallidus and increase motor asymmetry. Synapse 69:103–114
Narendran R, Tumuluru D, May MA, Chowdari KV, Himes ML, Fasenmyer K, Frankle WG, Nimgaonkar VL (2016) Cortical dopamine transmission as measured with the [11C] FLB 457–amphetamine PET imaging paradigm is not influenced by COMT genotype. PLoS One 11:e0157867
Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR (2015) The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci 16:305–312
O'Tuathaigh CMP, Clarke G, Walsh J, Desbonnet L, Petit E, O'Leary C, Tighe O, Clarke N, Karayiorgou M, Gogos JA, Dinan TG, Cryan JF, Waddington JL (2012) Genetic vs. pharmacological inactivation of COMT influences cannabinoid-induced expression of schizophrenia-related phenotypes. Int J Neuropsychopharmacol 15:1331–1342
Pelayo-Terán JM, Pérez-Iglesias R, Mata I, Carrasco-Marín E, Vázquez-Barquero JL, Crespo-Facorro B (2010) Catechol-O-methyltransferase (COMT) Val158Met variations and cannabis use in first-episode non-affective psychosis: clinical-onset implications. Psychiatry Res 179:291–296
Pistis M, Ferraro L, Pira L, Flore G, Tanganelli S, Gessa GL, Devoto P (2002) Δ 9-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res 948:155–158
Prescot AP, Renshaw PF, Yurgelun-Todd DA (2013) γ-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend 129:232–239
Radhakrishnan R, Skosnik PD, Cortes-Briones J, Sewell RA, Carbuto M, Schnakenberg A, Cahill J, Bois F, Gunduz-Bruce H, Pittman B (2015) GABA deficits enhance the psychotomimetic effects of Δ9-THC. Neuropsychopharmacology 40:2047–2056
Ranganathan M, Radhakrishnan R, Addy PH, Schnakenberg-Martin AM, Williams AH, Carbuto M, Elander J, Pittman B, Sewell RA, Skosnik PD (2017) Tetrahydrocannabinol (THC) impairs encoding but not retrieval of verbal information. Prog Neuro-Psychopharmacol Biol Psychiatry 79:176–183
Shafa R (2009) Poster 3: Comt-inhibitors may be a promising tool in treatment of marijuana addiction. Am J Addict 18:322
Sherif M, Radhakrishnan R, D’Souza DC, Ranganathan M (2016) Human laboratory studies on cannabinoids and psychosis. Biol Psychiatry 79:526–538
Spronk DB, Van der Schaaf ME, Cools R, De Bruijn ER, Franke B, van Wel JH, Ramaekers JG, Verkes RJ (2016) Acute effects of cocaine and cannabis on reversal learning as a function of COMT and DRD2 genotype. Psychopharmacology 233:199–211
Stokes PR, Mehta MA, Curran HV, Breen G, Grasby PM (2009) Can recreational doses of THC produce significant dopamine release in the human striatum? Neuroimage 48:186–190
Temple EC, Driver M, Brown RF (2014) Cannabis use and anxiety: is stress the missing piece of the puzzle? Front Psychol 5:168
Tunbridge EM, Dunn G, Murray RM, Evans N, Lister R, Stumpenhorst K, Harrison PJ, Morrison PD, Freeman D (2015) Genetic moderation of the effects of cannabis: catechol-O-methyltransferase (COMT) affects the impact of Δ9-tetrahydrocannabinol (THC) on working memory performance but not on the occurrence of psychotic experiences. J Psychopharmacol 29:1146–1151
Vaessen TSJ, de Jong L, Schäfer AT, Damen T, Uittenboogaard A, Krolinski P, Nwosu CV, Pinckaers FME, Rotee ILM, Smeets APW (2018) The interaction between cannabis use and the Val158Met polymorphism of the COMT gene in psychosis: a transdiagnostic meta–analysis. PLoS One 13:e0192658
Van der Knaap L, Schaefer J, Franken I, Verhulst F, van Oort F, Riese H (2014) Catechol-O-methyltransferase gene methylation and substance use in adolescents: the TRAILS study. Genes Brain Behav 13:618–625
Verrico CD, Jentsch JD, Roth RH (2003) Persistent and anatomically selective reduction in prefrontal cortical dopamine metabolism after repeated, intermittent cannabinoid administration to rats. Synapse 49:61–66
Vinkers CH, Van Gastel WA, Schubart CD, Van Eijk KR, Luykx JJ, Van Winkel R, Joels M, Ophoff RA, Boks MP, Genetic R, Investigators OUP, Bruggeman R, Cahn W, de Haan L, Kahn RS, Meijer CJ, Myin-Germeys I, van Os J, Wiersma D (2013) The effect of childhood maltreatment and cannabis use on adult psychotic symptoms is modified by the COMT Val(1)(5)(8)Met polymorphism. Schizophr Res 150:303–311
Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT (2007) Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci 27:10196–10209
Zammit S, Spurlock G, Williams H, Norton N, Williams N, O’DONOVAN MC, Owen MJ (2007) Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use. Br J Psychiatry 191:402–407
Acknowledgments
We also acknowledge support from the (1) Department of Veterans Affairs, (2) National Institute of Drug Abuse, and (3) National Institute of Alcoholism and Alcohol Abuse.
Funding
Funding and support from R01 DA12382 (Deepak Cyril D’Souza, M.D.), VA Connecticut Healthcare System (VACHS), NARSAD Young Investigator Award 2008 (Savita Bhakta, M.D.) and National Institutes of Mental Health (NIMH) R25 MH071584, “Integrated Mentored Patient-Oriented Research Training (IMPORT) in Psychiatry” (Joao P. De Aquino, M.D.), and Dana Foundation David Mahoney program and CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS) (Rajiv Radhakrishnan, M.D.). Deepak Cyril D’Souza has received in the past 3 years and currently receives research grant support administered through Yale University School of Medicine from Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Fig. S1
Study Design. Schedule of testing for sub-studies I and II. Sub-study I included 74 individuals of 3 genotypes (Val/Val, Val/Met, Met/Met). THC was administered at time point 0. Sub-study II included 22 homozygote (10 Val/Val, 22 Met/Met) individuals. Tolcapone was administered at time point -90 minutes. THC was administered at time point 0. Subjective and behavioral measures were collected before and after THC administration time points -30, 0 ,+60 and +300 minutes. Cognitive testing was done once approximately 30 minutes after THC administration. (PDF 157 kb)
ESM 1
(DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Ranganathan, M., De Aquino, J.P., Cortes-Briones, J.A. et al. Highs and lows of cannabinoid-dopamine interactions: effects of genetic variability and pharmacological modulation of catechol-O-methyl transferase on the acute response to delta-9-tetrahydrocannabinol in humans. Psychopharmacology 236, 3209–3219 (2019). https://doi.org/10.1007/s00213-019-05273-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05273-5