Abstract
Rationale
Corporeal awareness is an integral component of self-consciousness and is distorted in several neurological and psychiatric disorders. Research regarding the neural underpinnings of corporeal awareness has made much progress recently using the rubber hand illusion (RHI) procedure. However, more studies are needed to investigate the possibility of several dissociable constructs related to the RHI specifically, and corporeal awareness generally.
Objectives
Considering dopamine’s involvement in many perceptual-motor learning processes, as well as its apparent relationship with disorders such as schizophrenia that are linked to body ownership disturbances, we gave 0.45 mg/kg dexamphetamine (a dopamine transporter reverser) to 20 healthy participants to examine the effects of increased dopamine transmission on the RHI.
Methods
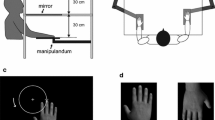
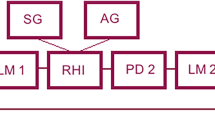
The effect of dexamphetamine on separate quantitative constructs underlying RHI were examined including embodiment of rubber hand, loss of ownership of real hand, perception of movement, affect, deafference, and proprioceptive drift. The experiment was a double-blind, placebo-controlled, cross-over design.
Results
Dexamphetamine increased participants’ ratings of embodiment (particularly “ownership”) of the rubber hand and was associated with the experience of loss of ownership of the person’s real hand. There were significant increases from asynchronous to synchronous stroking for the measures of movement and proprioceptive drift after placebo but not dexamphetamine. There were no changes in the measures of other constructs.
Conclusions
These results show a novel pharmacological manipulation of separate constructs of the RHI. This finding may aid in our understanding of disorders that have overlapping disturbances in both dopamine activity and body representations, particularly schizophrenia.





Similar content being viewed by others
References
Aglioti SM, Fiorio M, Forster B, Tinazzi M (2003) Temporal discrimination of cross-modal and unimodal stimuli in generalized dystonia. Neurology 60:782–785
Albrecht MA, Martin-Iverson MT, Price GW, Lee J, Iyyalol R (2010) Dexamphetamine reduction of P3a and P3b for auditory but not visual stimuli in healthy participants. J Psychopharmacol. doi:10.1177/0269881110376686
Almeida Q, Frank J, Roy E, Jenkins M, Spaulding S, Patla A et al (2005) An evaluation of sensorimotor integration during locomotion toward a target in Parkinson’s disease. Neurosci 134:283–293
Andringa G, Drukarch B, Leysen JE, Cools AR, Stoof JC (1999) The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur J Pharmacol 364:33–41
Angrist B, Gershon S (1970) The phenomenology of experimentally induced amphetamine psychosis. Biol Psychiatry 2:95–107
Armel KC, Ramachandran VS (2003) Projecting sensations to external objects: evidence from skin conductance response. Proc R Soc Lond B Biol Sci 270:1499–1506
Artieda J, Pastor MA, Lacruz F, Obeso JA (1992) Temporal discrimination is abnormal in Parkinson’s disease. Brain 115:199–210
Arzy S, Overney LS, Landis T, Blanke O (2006) Neural mechanisms of embodiment: asomatognosia due to premotor Cortex damage. Arch Neurol 63:1022–1025
Bergson C, Mrzljak L, Smiley J, Pappy M, Levenson R, Goldman-Rakic P (1995) Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci 15:7821–7836
Berlucchi G, Aglioti SM (1997) The body in the brain: neural bases of corporeal awareness. Trends Neurosci 20:560–564
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28:309–369
Blanke O, Arzy S (2005) The out-of-body experience: disturbed self-processing at the temporo-parietal junction. Neuroscientist 11:16–24
Blanke O, Metzinger T (2009) Full-body illusions and minimal phenomenal selfhood. Trends in Cognit Sci 13:7–13
Botvinick M, Cohen J (1998) Rubber hands ‘feel’ touch that eyes see. Nature 391:756
Bromage P, Melzack R (1974) Phantom limbs and the body schema. Can J Anesth 21:267–274
Buhusi CV, Meck WH (2002) Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci 116:291–297
Bütefisch CM, Davis BC, Sawaki L, Waldvogel D, Classen J, Kopylev L et al (2002) Modulation of use-dependent plasticity by d-amphetamine. Ann Neurol 51:59–68
Castner SA, Williams GV (2007) Tuning the engine of cognition: a focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogni 63:94–122
Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P (2001) Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci 13:1071–1077
Cepeda C, Buchwald NA, Levine MS (1993) Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A 90:9576–9580
Cooper SJ, Donald O (2005) Hebb’s synapse and learning rule: a history and commentary. Neurosci Biobehav Rev 28:851–874
Corbetta M, Patel G, Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–324
Corlett P, Honey G, Fletcher P (2007) From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol 21:238–252
Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V (2002) Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci 22:9320–9330
Coslett HB, Saffran EM, Schwoebel J (2002) Knowledge of the human body. Neurology 59:357–363
Crow TJ (1980) Molecular pathology of schizophrenia: more than one disease process? Br Med J 280:66–68
Czermak C, Lehofer M, Liebmann PM, Traynor J (2006) [35S]GTP[gamma]S binding at the human dopamine D4 receptor variants hD4.2, hD4.4 and hD4.7 following stimulation by dopamine, epinephrine and norepinephrine. Eur J Pharmacol 531:20–24
de Vignemont F (2010) Body schema and body image—pros and cons. Neuropsychologia 48:669–680
Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M (2003) Pharmacological modulation of perceptual learning and associated cortical reorganization. Science 301:91–94
Ehrsson HH (2009) How many arms make a pair? Perceptual illusion of having an additional limb. Perception 38:310
Ehrsson HH, Spence C, Passingham RE (2004) That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305:875–877
Ehrsson HH, Holmes NP, Passingham RE (2005) Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J Neurosci 25:10564–10573
Ehrsson HH, Wiech K, Weiskopf N, Dolan RJ, Passingham RE (2007) Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc Natl Acad Sci U S A 104:9828–9833
Farde L, Suhara T, Nyberg S, Karlsson P, Nakashima Y, Hietala J et al (1997) A PET-study of [11C]FLB 457 binding to extrastriatal D2-dopamine receptors in healthy subjects and antipsychotic drug-treated patients. Psychopharmacol 133:396–404
Farrer C, Frith C (2002) Experiencing oneself vs another person as being the cause of an action: the neural correlates of the experience of agency. Neuroimage 15:596–603
Farrer C, Franck N, Georgieff N, Frith C, Decety J, Jeannerod M (2003) Modulating the experience of agency: a positron emission tomography study. Neuroimage 18:324–333
Fletcher P, Frith C (2009) Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nat Rev Neurosci 10:48–58
Foucher J, Lacambre M, Pham B, Giersch A, Elliott M (2007) Low time resolution in schizophrenia: lengthened windows of simultaneity for visual, auditory and bimodal stimuli. Schizophr Res 97:118–127
Fox, J (2010) car: companion to applied regression, retrieved from http://CRAN.R-project.org/package=car
Gallagher S (2005) How the body shapes the mind. Oxford University Press USA, New York
Giersch A, Lalanne L, Corves C, Seubert J, Shi Z, Foucher J et al (2009) Extended visual simultaneity thresholds in patients with schizophrenia. Schizophr Bull 35:816–825
Goldman-Rakic P, Lidow M, Gallager D (1990) Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci 10:2125–2138
Goldsmith SK, Joyce JN (1996) Dopamine D2 receptors are organized in bands in normal human temporal cortex. Neurosci 74:435–451
Gurden H, Takita M, Jay TM (2000) Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci 20:RC106
Head H, Holmes G (1911) Sensory disturbances from cerebral lesions. Brain 34:102
Hebb DO (1949) The organisation of behaviour. Wiley, New York
Hegadoren KM, Greenshaw AJ, Baker GB, Martin-Iverson MT, Lodge B, Soin S (1994) 4-Ethoxyamphetamine: effects on intracranial self-stimulation and in vitro uptake and release of 3 H-dopamine and 3 H-serotonin in the brains of rats. J Psychiatry Neurosci 19:57–62
Heginbotham L, Dunwiddie T (1991) Long-term increases in the evoked population spike in the CA1 region of rat hippocampus induced by beta-adrenergic receptor activation. J Neurosci 11:2519–2527
Hollerman JR, Schultz W (1998) Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci 1:304–309
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Holmes NP, Spence C (2007) Dissociating body image and body schema with rubber hands. Behav Brain Sci 30:211–212
Holmes NP, Snijders HJ, Spence C (2006) Reaching with alien limbs: visual exposure to prosthetic hands in a mirror biases proprioception without accompanying illusions of ownership. Percept Psychophys 68:685–701
Hopkins W, Johnston D (1984) Frequency-dependent noradrenergic modulation of long-term potentiation in the hippocampus. Science 226:350–352
Hopkins W, Johnston D (1988) Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J Neurophysiol 59:667–687
Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR (2011) Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci 31:2481–2487
Howes OD, Kapur S (2009) The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull 35:549–562
Kammers MP, van der Ham I, Dijkerman H (2006) Dissociating body representations in healthy individuals: differential effects of a kinaesthetic illusion on perception and action. Neuropsychologia 44:2430–2436
Kammers MP, Verhagen L, Dijkerman HC, Hogendoorn H, De Vignemont F, Schutter DJLG (2009) Is this hand for real? Attenuation of the rubber hand illusion by transcranial magnetic stimulation over the inferior parietal lobule. J Cogn Neurosci 21:1311–1320
Kapur S (2003) Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 160:13–23
Kapur S, Mizrahi R, Li M (2005) From dopamine to salience to psychosis—linking biology, pharmacology and phenomenology of psychosis. Schizophr Res 79:59–68
Keijsers NLW, Admiraal MA, Cools AR, Bloem BR, Gielen CCAM (2005) Differential progression of proprioceptive and visual information processing deficits in Parkinson’s disease. Eur J Neuroscience 21:239–248
Koek W, Slangen JL (1983) Effects of d-amphetamine and morphine on discrimination: signal detection analysis and assessment of response repetition in the performance deficits. Psychopharmacol 80:125–128
Kubrusly RCC, Ventura ALM, de Melo Reis RA, Serra GCF, Yamasaki EN, Gardino PF et al (2007) Norepinephrine acts as D1-dopaminergic agonist in the embryonic avian retina: late expression of [beta]1-adrenergic receptor shifts norepinephrine specificity in the adult tissue. Neurochem Int 50:211–218
Lanau F, Zenner M, Civelli O, Hartman DS (2002) Epinephrine and norepinephrine act as potent agonists at the recombinant human dopamine D4 receptor. J Neurochem 68:804–812
Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J et al (1996) Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A 93:9235–9240
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R (1999) Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 46:56–72
Lawrence, MA (2010) ez: easy analysis and visualization of factorial experiments, http://CRAN.R-project.org/package=ez
Lee M, Kim H, Lyoo C (2005) "Off" gait freezing and temporal discrimination threshold in patients with Parkinson disease. Neurology 64:670–674
Li K, Pickett K, Nestrasil I, Tuite P, Konczak J (2010) The effect of dopamine replacement therapy on haptic sensitivity in Parkinson’s disease. J Neurol 257:1992–1998
Linden DEJ (2005) The P300: where in the brain is it produced and what does it tell us? Neuroscientist 11:563–576
Ljungberg T, Ungerstedt U (1976) Sensory inattention produced by 6-hydroxydopamine-induced degeneration of ascending dopamine neurons in the brain. Exp Neurol 53:585–600
Longo MR, Schüür F, Kammers MP, Tsakiris M, Haggard P (2008) What is embodiment? A psychometric approach. Cognition 107:978–998
MacNab MW, Foltz EL, Sweitzer J (1985) Evaluation of signal detection theory on the effects of psychotropic drugs on critical flicker-fusion frequency in normal subjects. Psychopharmacol 85:431–435
Martin SJ, Grimwood PD, Morris RGM (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Ann R Neurosci 23:649–711
Meck WH (1986) Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol Biochem Behav 25:1185–1189
Molina-Luna K, Pekanovic A, Röhrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti M et al (2009) Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS ONE 4:e7082
Morgan HL, Turner DC, Corlett P, Absalom AR, Adapa R, Arana FS et al (2010) Exploring the impact of the ketamine on the experience of illusory body ownership. Biol Psychiatry. doi:10.1016/j.biopsych.2010.07.032
Newman-Tancredi A, Audinot-Bouchez V, Gobert A, Millan MJ (1997) Noradrenaline and adrenaline are high affinity agonists at dopamine D4 receptors. Eur J Pharmacol 319:379–383
Nieuwenhuis S, Aston-Jones G, Cohen JD (2005) Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull 131:510–532
Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W (2004) Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cereb Cortex 14:1240–1245
Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F et al (2006) Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. Eur J Neurosci 23:1651–1657
Paillard J (1999) Body schema and body image—a double dissociation, in motor control today and tomorrow (Gantchev G, Mori S and Massion J eds). Academic, Sofia
Paqueron X, Leguen M, Rosenthal D, Coriat P, Willer JC, Danziger N (2003) The phenomenology of body image distortions induced by regional anaesthesia. Brain 126:702–712
Peled A, Ritsner M, Hirschmann S, Geva AB, Modai I (2000) Touch feel illusion in schizophrenic patients. Biol Psychiatry 48:1105–1108
Philips SR, Robson AM, Boulton AA (1982) Unstimulated and amphetamine-stimulated release of endogenous noradrenaline and dopamine from rat brain in vivo. J Neurochem 38:1106–1110
Pinheiro, J, Bates, D, DebRoy, S, Sarkar, D & R Core Team (2009) nlme: linear and nonlinear mixed effects models, http://cran.r-project.org/package=nlme
Rammsayer TH (1993) On dopaminergic modulation of temporal information processing. Biol Psychol 36:209–222
Rammsayer TH (1999) Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B 52:273–286
Rammsayer TH (2009) Effects of pharmacologically induced dopamine-receptor stimulation on human temporal information processing. NeuroQuantology 7:103–113
R Development Core Team (2010) R: a language and environment for statistical computing, r foundation for statistical computing, Vienna, Austria. http://www.R-project.org
Reynolds JNJ, Hyland BI, Wickens JR (2001) A cellular mechanism of reward-related learning. Nature 413:67–70
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18:247–291
Roitman MF, van Dijk G, Thiele TE, Bernstein IL (2001) Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behav Brain Res 122:193–199
Schneider JS, Diamond SG, Markham CH (1987) Parkinson’s disease: sensory and motor problems in arms and hands. Neurology 37:951–956
Schwoebel J, Coslett HB (2005) Evidence for multiple, distinct representations of the human body. J Cognit Neurosci 17:543–553
Seamans JK, Floresco SB, Phillips AG (1998) D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci 18:1613–1621
Seeman P (1987) Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1:133–152
Seitz AR, Dinse HR (2007) A common framework for perceptual learning. Curr Opin Neurobiol 17:148–153
Shen W, Flajolet M, Greengard P, Surmeier DJ (2008) Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321:848–851
Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM (1997) A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusions of alien control). Brain 120:1997–2011
Strube MJ, Bobko P (1989) Testing hypotheses about ordinal interactions: simulations and further comments. J Appl Psychol 74:247–252
Suppa A, Iezzi E, Conte A, Belvisi D, Marsili L, Modugno N et al (2010) Dopamine influences primary motor cortex plasticity and dorsal premotor-to-motor connectivity in Parkinson’s Disease. Cereb Cortex 20:2224–2233
Tsakiris M (2010) My body in the brain: a neurocognitive model of body-ownership. Neuropsychologia 48:703–712
Tsakiris M, Prabhu G, Haggard P (2006) Having a body versus moving your body: how agency structures body-ownership. Conscious Cogn 15:423–432
Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR (2007a) Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb Cortex 17:2235–2244
Tsakiris M, Schütz-Bosbach S, Gallagher S (2007b) On agency and body-ownership: phenomenological and neurocognitive reflections. Conscious Cogn 16:645–660
Tsakiris M, Costantini M, Haggard P (2008) The role of the right temporo-parietal junction in maintaining a coherent sense of one’s body. Neuropsychologia 46:3014–3018
Vallar G, Papagno C (2003) Pierre Bonnier’s (1905) cases of bodily “aschématie”. Classic cases in neuropsychology 2:147–170
Waelti P, Dickinson A, Schultz W (2001) Dopamine responses comply with basic assumptions of formal learning theory. Nature 412:43–48
Waters FA, Badcock JC (2010) First-rank symptoms in schizophrenia: reexamining mechanisms of self-recognition. Schizophr Bull 36:510–517
Waters F, Jablensky A (2009) Time discrimination deficits in schizophrenia patients with first-rank (passivity) symptoms. Psychiatry Res 167:12–20
Xu T, Yao W (2010) D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc Natl Acad Sci U S A 107:16366–16371
Zampini M, Moro V, Aglioti SM (2004) Illusory movements of the contralesional hand in patients with body image disorders. J Neurol Neurosurg Psychiatry 75:1626–1628
Acknowledgements
This research was funded by a National Health and Medical Research Council grant (ID: 254619). Matthew Albrecht is the recipient of a Clinical Neurophysiology supplementary scholarship from the Department of Neurophysiology, North Metropolitan Area Health Service—Mental Health and the School of Medicine and Pharmacology of the University of Western Australia. Flavie Waters is recipient of Australian National Health and Medical Research Council (NHMRC) grants (ID: 404117; 634328).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albrecht, M.A., Martin-Iverson, M.T., Price, G. et al. Dexamphetamine effects on separate constructs in the rubber hand illusion test. Psychopharmacology 217, 39–50 (2011). https://doi.org/10.1007/s00213-011-2255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2255-y