Abstract
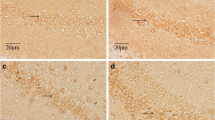
The present study was designed to evaluate the effects of chronic fluorosis on the dynamics (including fusion and fission proteins), fragmentation, and distribution of mitochondria in the cortical neurons of the rat brain in an attempt to elucidate molecular mechanisms underlying the brain damage associated with excess accumulation of fluoride. Sixty Sprague–Dawley rats were divided randomly into three groups of 20 each, that is, the untreated control group (drinking water naturally containing <0.5 mg fluoride/l, NaF), the low-fluoride group (whose drinking water was supplemented with 10 mg fluoride/l) and the high-fluoride group (50 mg fluoride/l). After 6 months of exposure, the expression of mitofusin-1 (Mfn1), fission-1 (Fis1), and dynamin-related protein-1 (Drp1) at both the protein and mRNA levels were detected by Western blotting, immunohistochemistry, and real-time PCR, respectively. Moreover, mitochondrial morphology and distribution in neurons were observed by transmission electron or fluorescence microscopy. In the cortices of the brains of rats with chronic fluorosis, the level of Mfn1 protein was clearly reduced, whereas the levels of Fis1 and Drp1 were elevated. The alternations of expression of the mRNAs encoding all three of these proteins were almost the same as the corresponding changes at the protein levels. The mitochondria were fragmented and the redistributed away from the axons of the cortical neurons. These findings indicate that chronic fluorosis induces abnormal mitochondrial dynamics, which might in turn result in a high level of oxidative stress.





Similar content being viewed by others
References
Aschrafi A, Natera-Naranjo O, Gioio AE, Kaplan BB (2010) Regulation of axonal trafficking of cytochrome c oxidase IV mRNA. Mol Cell Neurosci 43:422–430
Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R (2007) Mitochondrial bioenergetics and structural network organization. J Cell Sci 120:838–848
Bereiter-Hahn J, Jendrach M (2010) Mitochondrial dynamics. Int Rev Cell Mol Biol 284:1–65
Bereiter-Hahn J, Voth M (1994) Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech 27:198–219
Brooks C, Wei Q, Cho SG, Zheng D (2009) Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest 119:1275–1285
Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79–99
Cipolat S, de Brito OM, Zilio BD, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101:15927–15932
Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant opticatrophy. Nat Genet 26:207–210
Deng H, Dodson MW, Huang H, Guo M (2008) The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA 105:14503–14508
Exner N, Treske B, Paquet D, Holmstrom K, Schiesling C, Gispert S, Carballo-Carbajal I, Berg D, Hoepken HH, Gasser T, Kruger R, Winklhofer KF, Vogel F, Reichert AS, Auburger G, Kahle PJ, Schmid B, Haass C (2007) Loss-of-function of human PINK1 results in mitochondrial pathology and can be rescued by parkin. J Neurosci 27:12413–12418
Feng P, Wei J, Zhang Z (2011) Intervention of selenium on chronic fluorosis-induced injury of blood antioxidant capacity in rats. Biol Trace Elem Res 144:1024–1031
Gao Q, Liu YJ, Guan ZZ (2009) Decreased learning and memory ability in rats with fluorosis: increased oxidative stress and reduced cholinesterase activity in the brain. Fluoride 42:266–274
García-Montalvo EA, Reyes-Pérez H, Del Razo LM (2009) Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress. Toxicology 263:75–83
Guan ZZ, Wang YN, Xiao KQ, Dai DY, Chen YH, Liu JL, Sindelar P, Dallner G (1998) Influence of chronic fluorosis on membrane lipids in rat brain. Neurotoxicol Teratol 20:537–542
Guan ZZ, Xiao KQ, Zeng XY, Long YG, Cheng YH, Jiang SF, Wang YN, Liu JL, Dallner G (2000) Changed cellular membrane lipid composition and lipid peroxidation of kidney in rats with chronic fluorosis. Arch Toxicol 74:602–608
Hassunuma RM, Zen Filho EV, Ceolin DS, Cestari TM, Taga R, de Assis GF (2007) Ultrastructural and immunohistochemical study of the influence of fluoride excess on the development of rat incisor tooth buds. J Appl Oral Sci 15:292–298
He YQ, Pan Y, Ying LJ, Zhao R (2012) Differential expression ESTs associated with fluorosis in rats liver. Comp Funct Genomics 2012:208390
Izquierdo-Vega JA, Sánchez-Gutiérrez M, Del Razo LM (2008) Decreased in vitro fertility in male rats exposed to fluoride-induced oxidative stress damage and mitochondrial transmembrane potential loss. Toxicol Appl Pharmacol 230:352–357
Li Z, Okamoto K, Hayashi Y, Sheng M (2004) The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119:873–887
Liu YJ, Gao Q, Wu CX, Guan ZZ (2010) Alternations of nAChRs and ERK1/2in the brains of rats with chronic fluorosis and their connections with the decreased capacity of learning and memory. Toxicol Lett 192:324–329
Liu YJ, Guan ZZ, Gao Q, Pei JJ (2011) Increased level of apoptosis in rat brains and SH-SY5Y cells exposed to excessive fluoride—a mechanism connected with activation JNK phosphorylation. Toxicol Lett 204:183–189
Long YG, Wang YN, Chen J, Jiang SF, Nordberg A, Guan ZZ (2002) Chronic fluoride toxicity decreases the number of nicotinic acetylcholine receptors in rat brain. Neurotoxicol Teratol 24:751–757
Matsumoto S, Uchiumi T, Tanamachi H, Saito T, Yagi M, Takazaki S, Kanki T, Kang D (2012) Ribonucleoprotein Y-box-binding protein-1 regulates mitochondrial oxidative phosphorylation (OXPHOS) protein expression after serum stimulation through binding to OXPHOS mRNA. Biochem J 443:573–584
Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, Wood NW, Willems PH, Smeitink JA, Cookson MR, Bandmann O (2008) Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol 64:555–565
Mukhopadhyay P, Horváth B, Zsengellėr Z, Bátkai S, Cao S, Kechris M, Holovac E, Erdėlyi K, Tanchian G, Liaudet L, Stillman IE, Joseph J, Kalyanaraman B, Pacher P (2012) Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially-targeted antioxidants. Free Radic Biol Med 53:1123–1138
Mullenix PJ, Denbesten PK, Schunior A, Kernan WJ (1995) Neurotoxicity of sodium fluoride in rats. Neurotoxicol Teratol 17:169–177
Okamoto K, Shaw JM (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39:503–536
Park J, Lee G, Chung J (2009) The PINK1-Parkin pathway is involved in the regulation of mitochondrial remodeling process. Biochem Biophys Res Commun 378:518–523
Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ (2008) The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA 105:1638–1643
Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C (2010) New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci 123:2533–2542
Shan KR, Qi XL, Long YG, Nordberg A, Guan ZZ (2004) Decreased nicotinic receptors in PC12 cells and rat brains influenced by fluoride toxicity—a mechanism relating to a damage at the level in post-transcription of the receptor genes. Toxicology 200:169–177
Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ (2005) Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47:365–378
Wang YN, Xiao KQ, Liu JL, Dallner G, Guan ZZ (2000) Effect of long term fluoride exposure on lipid composition in rat liver. Toxicology 146:161–169
Wang H, Lim PJ, Karbowski M, Monteiro MJ (2009a) Effects of overexpression of huntingtin proteins on mitochondrial integrity. Hum Mol Genet 18:737–752
Wang X, Su B, Lee H, Li X, Perry G, Smith MA, Zhu X (2009b) Impaired balance of mitochondria fission and fusion in Alzheimer disease. J Neurosci 29:9090–9103
Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW II, O’Rourke B, Kitsis RN (2012) Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci USA 109:6566–6571
Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA 105:7070–7075
Zhang CY, Xu SG, Huang XX (2005) A novel and convenient relative quantitative method of fluorescence real-time PCR assay based on slope of standard curve. Pro Biochem Biophys 32:883–889
Zhu XW, Rottkamp CA, Boux H, Takeda A, Perry G, Smith MA (2000) Activation of p38 kinase links tau phosphorylation, oxidative stress, and cell cycle-related events in Alzheimer disease. J Neuropathol Exp Neurol 59:880–888
Acknowledgments
This work was financed by grants from the Natural Science Foundation of China (81160335), the Foundation of the Ministry of Science and Technology of China (2010DFB30530, 2011BAZ03220), and the Governmental Foundation at Guizhou Province, China.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, DD., Guan, ZZ., Liu, YJ. et al. The influence of chronic fluorosis on mitochondrial dynamics morphology and distribution in cortical neurons of the rat brain. Arch Toxicol 87, 449–457 (2013). https://doi.org/10.1007/s00204-012-0942-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-012-0942-z