Abstract
The Fusarium head blight of grain cereals is a significant disease worldwide. In Argentina, high levels of contamination with Fusarium proliferatum have been found in crops. Many strains of the Pseudomonas genus antagonize the growth of fungi by different mechanisms, such as the production of antibiotics, siderophores, volatiles, and extracellular enzymes. In this work, we have designed a new system for studying the growth inhibition of F. proliferatum—namely by volatile compounds produced by Pseudomonas fluorescens MGR12. In both rich and minimal media, the bacterium released volatiles that negatively affected the mycelial growth of that phytopathogenic fungus. These bacterial compounds were analyzed by gas chromatography–mass spectrometry, but only a few could be identified by comparing their mass spectra with the libraries of the National Institutes of Standards and Technology MS search.
Similar content being viewed by others
Introduction
The Fusarium head blight of grain cereals is a significant disease throughout the world, with more than 20 Fusarium species having been identified as etiologic agents. In addition, the genus Fusarium produces mycotoxins, called fumonisins, that are hazardous to both animal and human health (Chulze et al. 1996; Edwards 2004). If infected cereal grain is stored or transported with too high moisture content, a postharvest growth of the fungus occurs along with an increase in the levels of the mycotoxin (Magan et al. 2010). In Argentina, high levels of contamination with Fusarium species have been found in wheat grain, with F. proliferatum being the most frequently isolated species. Natural contamination with fumonisin has also been found in wheat grain, but the level depended on the amount of rainfall during the wheat-cultivation periods (Palacios et al. 2011).
Many strains of the Pseudomonas genus have been studied as biological control agents against soilborne plant pathogens. The traits that make Pseudomonas spp. an effective potential fungal antagonist are the ability to grow rapidly in vitro, quickly utilize root exudates, colonize and multiply in the rhizosphere, compete aggressively with other microorganisms, adapt to environmental stresses, and produce a wide spectrum of bioactive metabolites, e.g., antibiotics, siderophores, volatiles, and extracellular enzymes (Weller 2007; Fischer et al. 2013).
In recent years, volatile organic compounds (VOCs) have begun to receive special consideration within the biocontrol scenario. These compounds are small organic molecules (of molecular mass usually <300 Da) that characteristically have a high vapor pressure and are therefore easily volatilized. Some VOCs produced by rhizobacteria have an antifungal potential and can act over long distances via diffusion in air and through soil pores (Wheatley 2002; Kai et al. 2007). A given VOC produced by a particular bacterial strain does not cause the same effects or the same degree of inhibition in all fungi; rather, the responses depend on the specific fungus–bacterium combination. These specificities may occur because not all fungi respond to the same VOCs, the sites of action may be different, or the fungi might possess different abilities to detoxify a given volatile metabolite. In this regard, rhizobacteria nevertheless produce different VOCs, depending on the growth medium and conditions (Kai et al. 2009). Carbon and nitrogen sources increase VOC production: for example, tryptic soy agar—which is richer in carbon and nitrogen compounds than nutrient agar medium (NA)—induces more VOC production and results in greater antifungal activity (Fernando et al. 2005).
In this report, we document the ability of Pseudomonas fluorescens MGR12—a native strain isolated from the corn plant (Cordero et al. 2012)—to produce VOCs involved in the biocontrol of F. proliferatum.
Materials and methods
Bacterial and fungal strains and growth conditions
The strains of the genus Pseudomonas used in this study belong to the collection of our laboratory, having been originally isolated from the rhizosphere of corn (Cordero et al. 2012). The bacterial strains were routinely cultured on nutrient agar medium (Britania, Argentina) at 30 °C. Fusarium proliferatum RC 479 (provided by Mycology of the Universidad Nacional de Río Cuarto) was routinely grown on carnation leaf agar (Fisher et al. 1982) at 24 °C.
Screening for antifungal volatile-producing bacteria: the divided-plate method
The production of antifungal volatile compounds by strains of Pseudomonas spp. was analyzed as described by Fernando et al. (2005) with some modifications. A fresh plug of mycelium from F. proliferatum RC 479 was placed onto one half of a divided plate containing potato dextrose agar (PDA) medium, and the bacterial strain was streaked onto the other half of the divided plate. The plates were immediately wrapped in ParafilmTM to prevent the escape of volatile compounds and incubated at 25 °C. Plates containing only F. proliferatum and likewise wrapped with Parafilm were used as controls for fungal growth. In addition, plates cultivated with the phytopathogenic fungus and the same bacterial strain, but not wrapped with Parafilm, were included as controls to test the volatile nature of the inhibitory compounds.
Pseudomonas fluorescens strain MGR12 was selected for further analysis of VOCs because this strain showed the strongest inhibition against F. proliferatum RC 479.
Antifungal activity of the VOCs produced by strain MGR12 in liquid media
We designed a system of two kitasato flasks connected by a three-way stopcock, in order to demonstrate the inhibition of mycelial growth by volatile compounds produced by a bacterium growing in a liquid medium (Fig. 1). The strain MGR12 was cultured with shaking in 75 ml of trypticase soya broth (TSB) or glucose minimal medium (GMM), contained in the first filter flask with a rubber stopper, at 30 °C for 48 h. Before the onset of incubation, the output of that flask was connected to the second filter flask through a three-way stopcock that initially remained closed. After incubation, a fresh plug of mycelium was placed in the second filter flask containing solid PDA medium, and then, the three-way stopcock was opened to allow the flow of volatiles from first to the second filter flask. The two cultures were then incubated with agitation at 30 °C for 9 days.
As a control, an identical incubation was performed in parallel, but with the first filter flask containing TSB or GMM medium alone without bacterial inoculation.
The mycelial growth in the presence of the bacterial volatiles was compared to that of the control. The experiment was performed in triplicate and repeated twice.
Isolation and identification of VOCs
The volatiles produced by the strain MGR12 were collected from the headspace of bacterial cultures as described by Fernando et al. (2005) and Kai et al. (2010) with some modifications. The strain was cultured with shaking in 75 ml of TSB or GMM media in a filter flask fitted with a rubber stopper through which a sterile 0.4-cm-diameter glass tubing with a cotton plug was inserted to within 1 cm of the surface of the bacterial broth below. The efferent arm of the filter flask was connected to a trap for volatiles consisting in a plug containing 150 mg of active charcoal. The entire system—wrapped hermetically in Parafilm to prevent the escape of VOCs—was incubated at 30 °C with agitation for 48–72 h.
After the incubation, the volatile compounds were collected into the charcoal trap through the use of two pumps: One, producing a constant positive pressure of influent air, was connected to the upper glass tube; another, removing the air enriched in volatiles through a constant negative pressure, was connected to the efferent end of the volatile trap. A filter flask containing bacteria-free TSB or GMM medium was sampled as a control.
The compounds were eluted from the charcoal with 0.5 ml of methylene chloride.
Gas chromatography–mass spectrometry (GC–MS) analysis
The eluted samples were analyzed by GC–MS, Clarus 600 from Perkin Elmer. Turbo Mass software was used to acquire and control data from the GC–MS. All the separations were conducted through a Perkin Elmer fused silica Elite DB-5 capillary column (60 m; ID, 0.25 mm; film thickness, 0.25 mm) with helium as a carrier gas (49.6 psi). One milliliter of the sample was injected in the split injection mode (at 20 ml/min).
The following gas chromatographic and mass spectrometric detection conditions were used:
The oven-temperature program started with an initial temperature of 40 °C (held for 3 min) and then rose at 4 °C/min to 200 °C, with a column head pressure of 15 psi and injector and FID detector temperatures of 250 °C.
The GC transfer line was maintained at 200 °C, and the ionization carried out in the mass spectrometer under vacuum by electron impact with a −70-eV ionization energy. The chromatograms were run in the “scan” mode scanning the quadrupole from an m = z 50 to an m = z 300 (scan time: 0.2 s, interscan time: 0.1 s).
The volatile compounds were tentatively assigned by comparing their mass spectra with those of the libraries of the National Institutes of Standards and Technology (NIST) MS search 2.0 software.
Results
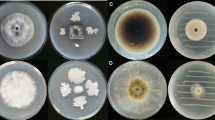
Inhibition of F. proliferatum by volatile compounds in divided-plate culture
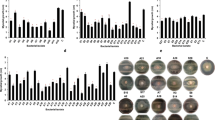
Six strains of from our collection of Pseudomonas spp. (Cordero et al. 2012) were screened for the production of antifungal volatiles in divided PDA plates. To that end, a dual-culture system was developed whereby the two organisms grew on the same plate but divided by a physical barrier so that volatiles, but not solutes of the bacteria, could reach the fungus (Kai et al. 2007).
In the dual-culture system, the growth of F. proliferatum was compared with the control (i.e., Parafilm-sealed plates with only fungus). All the strains tested produced volatile compounds that negatively affected the mycelial growth of F. proliferatum, but the degree of inhibition depended on the particular Pseudomonas strain, with P. fluorescens strain MGR12 being the most relevant as a potential biocontrol agent. A plateau in the VOCs emitted by strain MGR12 was reached between the 3rd and 4th day after inoculation in solid medium. Mycelial growth was not affected in plates unsealed with Parafilm or in sealed plates without bacteria present (controls; Fig. 2).
Inhibition of F. proliferatum by volatile compounds produced by strain MGR12 in liquid medium
To demonstrate that the inhibition of F. proliferatum also occurred when the bacterium grew in liquid medium, we designed a system of two-filter flasks in tandem connected by three-way stopcock. The strain MGR12 was grown in the first filter flask on a minimal (GMM) or a rich (TSB) medium for 48 h. The bacterial volatiles were then released into second filter flask containing fungal plug as described in Materials and Methods. In both growth media, strain MGR12 synthesized volatile compounds that inhibited the mycelial growth of F. proliferatum RC 479. Neither medium GMM nor TSB in the absence of bacteria produced any volatiles that suppressed fungal growth (Fig. 3).
Inhibition of fungal mycelial growth by volatile compounds produced by bacteria growing in liquid medium. In each of the pairs of filter flasks shown in the figure, F. proliferatum was grown in the right flask containing PDA medium and connected to the left flask containing a only TSB or b GMM medium both in the absence of bacteria, or c TSB or d GMM medium inoculated with P. fluorescens strain MGR12. The experiment shown here is representative of the results of three such experiments with each one performed in triplicate
Identification of volatile compounds
The profile of volatiles emitted by the strain MGR12 growing on minimal and rich media was analyzed by GC–MS. On GMM medium, the bacteria produced 33 different compounds, some of which volatiles could be identified by comparing their mass spectra with the libraries accessed by an NIST MS search: Those volatiles consisted of alkanes, alkenes, cycloalkanes, aromatics, amines, and phenolic and sulfur compounds (Fig. 4). In addition, only two compounds—having retention time of 4.49 and 35.74—were detected in the uninoculated medium.
Chromatographic profiles of volatiles emitted by P. fluorescens MGR12 on a GMM and c TSB medium as compared with uninoculated b GMM and d TSB medium alone. The VOCs identified by mass spectrometry were as follows: (1) 2-methyl-1-pentene, (2) 1,3,5-cycloheptatriene, (3) ethylbenzene, (4) 1,2-dimethylbenzene, (5) phenol, (6) 2,6,6-trimethyl-bicyclo [3.1.1] hept-3-ylamine, (7) 1-propene-1-thiol, (8) 1-methylene-1H-indene, (9) tridecane, (10) 2,6,10,14-tetramethylpentadecane, (11) tetradecane, (12) 2,6,11,15-tetramethylhexadecane, (13) cyclohexane, (14) ethanedioic acid, bis (trimethylsilyl) ester, (15) 6,7-dimethoxy-2-methyl-3,4-dihydro[1-D]isoquinoliniumion, (16) 2,4-dimethyl-1-heptene, (17) 1-undecene, (18) 1,4-dimethyl-benzene, (19) benzothieno[2,3-C]quinolin-6(5H)-one-2-methoxy, (20) decane, (21) dodecane, (22) tetramethylsilane, (23) tetradecane, and (24) hexadecane. * Unidentified volatile of retention time of 35.03
The results were more complex with TSB medium because it emitted several organic volatiles independently of the presence of bacteria. Of all the compounds detected on TSB medium, only 13 were produced by strain MGR12. The mass spectroscopic analysis allowed the tentative assignment of only a few of these compounds—namely 6,7-dimethoxy-2-methyl-3,4-dihydro(1-d)isoquinoliniumion, 1-undecene, benzothieno[2,3-c]quinolin-6(5H)-one-2-methoxy, and tetramethylsilane (Fig. 4).
A comparison of the volatiles released by P. fluorescens MGR12 on GMM and TSB medium indicated only three compounds common to both chromatograms (1,3,5-cycloheptatriene, tetradecane, and an unidentified volatile with a retention time of 35.03). Since, however, the first two of those was also emitted in uninoculated TSB medium, we speculate that an unidentified volatile compound (Fig. 5) could be involved in the biocontrol observed in these experiments.
Discussion
In the last decade, the bacterial volatiles have begun to have relevance as compounds involved in the biocontrol of phytopathogenic fungi. For example, the volatiles produced by different species of the Pseudomonas genus isolated from canola root and stubble and from soybean roots inhibited survival (sclerotia), infection (mycelia), and reproductive structures (ascospores) production of Sclerotinia sclerotiorum (Fernando et al. 2005). Kai et al. (2007) furthermore demonstrated that P. fluorescens L13-6-12 and P. trivialis 3Re2-7 emitted complex blends of volatiles that drastically inhibited the growth of Rhizoctonia solani. An analysis of the VOC spectra of those strains revealed that certain compounds are isolate-specific while others are emitted by both antagonists. In addition, unidentified volatile compounds from Bacillus subtilis caused morphologic abnormalities in several fungi, including Fusarium oxysporum and Alternaria alternata (Chaurasia et al. 2005). Moreover, Vespermann et al. (2007) demonstrated that the volatiles of Stenotrophomonas, Serratia, and Bacillus species inhibited mycelial growth of many fungi, and the strength of fungal growth arrest depended on the rhizobacterial isolate. Finally, Mackie and Wheatley (1999) have suggested that the fungal response to bacterial volatiles appears to be species-, environment-, and age-specific.
In the present study, six native strains of Pseudomonas were screened for the production of antifungal volatiles. In all instances, the mycelial growth was inhibited when the bacteria and fungi were grown in plates divided by a physical barrier and sealed with Parafilm, but no antifungal effect occurred in unsealed systems. This result strongly implies that the nature of those antifungal compounds is volatile.
Pseudomonas fluorescens MGR12 was the most potent biocontrol agent. The volatiles emitted by this strain were the fastest in inhibiting the mycelial growth in solid medium. For this reason, all subsequent experiments were realized with that same strain.
A system of two-filter flasks connected by a three-way stopcock was designed for studying the production of VOCs by the strain MGR12, growing on both rich and minimal liquid media, and the relevance of those compounds to biocontrol. During growth in both media, the bacteria released volatiles that suppressed the growth of F. proliferatum. These bacterial VOCs were analyzed by GC–MS, but only a few volatiles could be identified by comparing their mass spectra with those of the libraries of the NIST MS search. Similar results had been described by Kai et al. (2007), who identified by GC–MS only 20 VOCs out of approximately 80 that were emitted from the seven bacterial isolates tested. The authors suggested that those compounds neither were among the common VOCs previously identified in plants, animals, or bacteria, nor were present in the library of the NIST (comprising some 147,000 compounds). This provides a possible source of new, yet unknown small molecular mass chemicals (Kai et al. 2007). Nevertheless, such volatile mixtures emitted from bacteria have already been applied, but with the bioactive compound(s) still remaining undetermined (Kai et al. 2009).
Although rich media have frequently been used for studying bacterial VOCs, we detected the emission of many volatiles by the uninoculated TSB medium, whereas only two unidentified peaks were found to be emitted by the uninoculated GMM minimal medium. A comparison of the antifungal volatiles emitted by strain MGR12 in both media indicated only three compounds—i.e., 1,3,5-cycloheptatriene, tetradecane, and an unidentified volatile of retention time of 35.03—to be common to both TSB and GMM medium, but the first two of these VOCs were also released by the TSB medium alone without any cells present. We therefore believe that the volatile with a retention time of 35.03 could be involved in the biocontrol of F. proliferatum. This compound still remains to be identified.
We previously demonstrated that P. fluorescens MGR12 inhibited the growth of many phytopathogenic fungi—such as Fusarium verticillioides, Sclerotinia sclerotiorum, Sclerotinia minor, and Sclerotium rolfsii—but not F. proliferatum. Those earlier antifungal activities, however, were correlated with the production of lytic enzymes, siderophores, or hydrogen cyanide (Cordero et al. 2012). The present results with the strain MGR12 introduce a new mode of biocontrol action against F. proliferatum, a fungus causing significant economic losses in crops.
To our knowledge, the present work constitutes the first example of the biocontrol of F. proliferatum through contact with volatile compounds.
The findings of this study should expand our understanding of the possibilities for the biocontrol of Pseudomonas strains isolated from this region. The volatiles produced by the native strain P. fluorescens MGR12 could be employed for the protection of crops against F. proliferatum both here and elsewhere, thus at the same time reducing the need to employ the chemical fumigants that are so increasingly harmful to the environment.
Abbreviations
- VOCs:
-
Volatile organic compounds
- PDA:
-
Potato dextrose agar medium
- TSB:
-
Trypticase soya broth
- GMM:
-
Glucose minimal medium
- NA:
-
Nutrient agar medium
References
Chaurasia B, Pandey A, Palni LMS, Trivedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol Res 160:75–81
Chulze SN, Ramirez ML, Farnochi MC, Pascale M, Visconti A, March G (1996) Fusarium and fumonisins occurrence in Argentinean corn at different ear maturity stages. J Agric Food Chem 44:2797–2801
Cordero P, Cavigliasso A, Príncipe A, Godino A, Jofré E, Mori G, Fischer S (2012) Genetic diversity and antifungal activity of native Pseudomonas isolated from maize plants grown in a central region of Argentina. Syst Appl Microbiol 35:342–351
Edwards SG (2004) Influence of agricultural practices on Fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol Lett 153(1):29–35
Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964
Fischer S, Príncipe A, Alvarez F, Cordero P, Castro M, Godino A, Jofré E, Mori G (2013) Fighting plant diseases through the application of Bacillus and Pseudomonas strains. In: Aroca R (ed) Symbiotic endophytes, soil biology 37. Springer, Berlin, Heidelberg, pp 165–193
Fisher NL, Burgess LW, Toussoun TA, Nelson PE (1982) Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology 72:151–153
Kai M, Effmert U, Berg G, Piechulla B (2007) Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol 187:351–360
Kai M, Haustein M, Molina F, Petri A, Scholz B, Piechulla B (2009) Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 81:1001–1012
Kai M, Crespo E, Cristescu SM, Harren FJM, Francke W, Piechulla B (2010) Serratia odorifera: analysis of volatile emission and biological impact of volatile compounds on Arabidopsis thaliana. Appl Microbiol Biotechnol 88:965–976
Mackie AE, Wheatley RE (1999) Effects of the incidence of volatile organic compound interactions between soil bacterial and fungal isolates. Soil Biol Biochem 31:375–385
Magan N, Aldred D, Mylona K, Lambert RJW (2010) Limiting mycotoxins in stored wheat. Food Addit Contam 27:644–650
Palacios SA, Ramirez ML, Cabrera Zalazar M, Farnochi MC, Zappacosta D, Chiacchiera SM, Reynoso MM, Chulze SN, Torres AM (2011) Occurrence of Fusarium spp. and fumonisin in durum wheat grains. J Agric Food Chem 59(22):12264-12269
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microbiol 73(17):5639–5641
Weller DM (2007) Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256
Wheatley RE (2002) The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 81:357–364
Acknowledgments
The authors thank the laboratory of Mycology of the Universidad Nacional de Río Cuarto for providing strain F. proliferatum RC 479. We are also grateful to M. Palacio for helping with GC-MS analysis and D.F. Haggerty, a retired career investigator and native English speaker, for editing the final version of the manuscript. S. Fischer and E. Jofré are members of the Scientific Researcher Career-CONICET (National Council of Technological Researchs). P. Cordero has a doctoral fellowship from CONICET-Ministerio de Ciencia y Tecnología de Córdoba. This research was supported by SECYT of the Universidad Nacional de Río Cuarto, PIP-CONICET and Ministerio de Ciencia y Tecnología de Córdoba.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Cordero, P., Príncipe, A., Jofré, E. et al. Inhibition of the phytopathogenic fungus Fusarium proliferatum by volatile compounds produced by Pseudomonas . Arch Microbiol 196, 803–809 (2014). https://doi.org/10.1007/s00203-014-1019-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-014-1019-6