Abstract
Summary
Osteocyte is the orchestrator of bone remolding and decline in osteocyte autophagy is involved in senile osteoporosis. Our results suggested that rapamycin, at least in part by activating osteocyte autophagy, reduced the severity of age-related bone changes in trabecular bone of old male rats.
Introduction
Previous literatures have showed that osteocyte is the orchestrator of bone remolding and age-related decline in osteocyte number is associated with senile osteoporosis. Autophagy is an important cellular protective mechanism which can preserve osteocyte viability and failure of autophagy in osteocyte with age has been linked to senile osteoporosis. The purpose of this study was to explore whether rapamycin, one activator of autophagy, has protective effects on senile osteoporosis through inducing osteocyte autophagy.
Methods
Fifty-two 24-month-old male Sprague-Dawley (SD) rats were randomly divided into two groups. Rapamycin (1 mg/kg weight/day) or DMSO vehicle control was administered intraperitoneally for 12 weeks. BMD and bone microstructure were determined by Micro-CT. Fluorochrome labeling of the bones was performed to measure the mineral apposition rate (MAR). TRAP staining was performed to evaluate osteoclast number. The plasma levels of bone turnover markers were also analyzed. The effects of rapamycin on osteocyte autophagy were determined by immunohistochemistry, Western blot, and q-PCR. TUNEL was used to determine the prevalence of osteocyte apoptosis.
Results
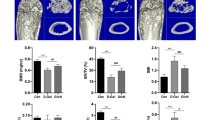
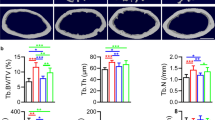
Micro-CT evaluation demonstrated that rapamycin had a protective effect on age-related bone loss in trabecular bone. Besides, rapamycin resulted in an obvious increase of MAR and a decrease of osteoclast number in contrast to the control group. Furthermore, rapamycin also induced autophagy in osteocyte demonstrated by increased LC3-positive osteocyte and increased LC3 turnover. In addition, rats treated with rapamycin exhibited decreased apoptosis of osteocyte determined by TUNEL.
Conclusions
These results suggested that rapamycin, at least in part by activating osteocyte autophagy, reduced the severity of age-related bone changes in trabecular bone of old male rats. Therefore, rapamycin might be a feasible therapeutic approach for senile osteoporosis.




Similar content being viewed by others
References
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with The International Osteoporosis Foundation (IOF) and the European Federation Of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8(1-2):136
Manolagas SC, Parfitt AM (2010) What old means to bone. Trends Endocrinol Metab 21(6):369–374
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94(1):25–34
Qiu S, Rao DS, Palnitkar S, Parfitt AM (2003) Reduced iliac cancellous osteocyte density in patients with osteoporotic vertebral fracture. J Bone Miner Res 18(9):1657–1663
Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem 282(37):27285–27297
Kogianni G, Mann V, Noble BS (2008) Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res 23(6):915–927
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42
Choi AM, Ryter SW, Levine B (2013) Autophagy in human health and disease. N Engl J Med 368(7):651–662
Xia X, Kar R, Gluhak-Heinrich J, Yao W, Lane NE, Bonewald LF, Biswas SK, Lo WK, Jiang JX (2010) Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res 25(11):2479–2488
Onal M, Piemontese M, Xiong J, Wang Y, Han L, Ye S, Komatsu M, Selig M, Weinstein RS, Zhao H, Jilka RL, Almeida M, Manolagas SC, O'Brien CA (2013) Suppression of autophagy in osteocytes mimics skeletal aging. J Biol Chem 288(24):17432–17440
Chen K, Yang YH, Jiang SD, Jiang LS (2014) Decreased activity of osteocyte autophagy with aging may contribute to the bone loss in senile population. Histochem Cell Biol 142(3):285–295
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O'Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36(6):585–595
Carames B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M (2012) Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis 71(4):575–581
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460(7253):392–395
Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One 5(4):e9979
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28(1):2–17
Wang L, Banu J, McMahan CA, Kalu DN (2001) Male rodent model of age-related bone loss in men. Bone 29(2):141–148
Pietschmann P, Skalicky M, Kneissel M, Rauner M, Hofbauer G, Stupphann D, Viidik A (2007) Bone structure and metabolism in a rodent model of male senile osteoporosis. Exp Gerontol 42(11):1099–1108
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26(2):229–238
Rochefort GY, Pallu S, Benhamou CL (2010) Osteocyte: the unrecognized side of bone tissue. Osteoporos Int 21(9):1457–1469
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17(10):1231–1234
Noble BS, Stevens H, Loveridge N, Reeve J (1997) Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone 20(3):273–282
Cejka D, Hayer S, Niederreiter B, Sieghart W, Fuereder T, Zwerina J, Schett G (2010) Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum 62(8):2294–2302
Smink JJ, Tunn PU, Leutz A (2012) Rapamycin inhibits osteoclast formation in giant cell tumor of bone through the C/EBPbeta - MafB axis. J Mol Med 90(1):25–30
Westenfeld R, Schlieper G, Woltje M, Gawlik A, Brandenburg V, Rutkowski P, Floege J, Jahnen-Dechent W, Ketteler M (2011) Impact of sirolimus, tacrolimus and mycophenolate mofetil on osteoclastogenesis—implications for post-transplantation bone disease. Nephrol Dial Transplant 26(12):4115–4123
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, D., Ren, H., Li, T. et al. Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos Int 27, 1093–1101 (2016). https://doi.org/10.1007/s00198-015-3325-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3325-5