Abstract
Introduction
Transvaginal mesh usage has been at the forefront of popular media and academic debate for the past 10 years. Several US Food and Drug Administration (FDA) communications, society statements, and research articles have been written in an attempt to define and articulate the classification system, safety data, and efficacy of this approach to transvaginal surgery. In this review, we explore the history of transvaginal mesh surgery for pelvic organ prolapse (POP), review FDA and society statements, and research current practice in the United States.
Methods
We searched the English language literature using PubMed for articles related to safety and monitoring of transvaginal mesh and reviewed all FDA publication and notices and gynecology and urogynecology society statements on its use in the United States. We then reviewed 22 articles and grouped them into several sections.
Results
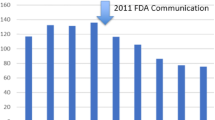
Mesh used to augment transvaginal repair of POP was introduced in the United States in 2005 without clinical safety and efficacy data. In the subsequent years of use, both major and minor complications were increasingly reported, leading to several FDA notifications and warnings. The type of mesh used, reporting and classifications systems, and provider usage has varied widely over time.
Conclusion
We present a historical review of transvaginal mesh use for pelvic organ prolapse in the United States from 2005 to 2016. There continues to be heated debate among practitioners about balancing the efficacy of mesh use to decrease recurrent prolapse and complications. Research into safety and efficacy, along with tighter FDA regulations, is ongoing.
Similar content being viewed by others
References
Bako A, Dhar R. Review of synthetic mesh-related complications in pelvic floor reconstructive surgery. Int Urogynecol J. 2009;20:103–11.
ACOG Committee Opinion No.513. Vaginal placement of synthetic mesh for pelvic organ prolapse. 2011.
Trabuco EC, Gebhart J. Up to Date, overview of transvaginal placement of reconstructive materials (surgical mesh or biografts) for treatment of pelvic organ prolapse or stress urinary incontinence. 2014. http://www.uptodate.com.proxy.uchicago.edu/contents/overview-of-transvaginal-placement-of-mesh-for-prolapse-and-stress-urinary-incontinence?source=search_result&search=Overview+of+transvaginal+placement+of+reconstructive+materials+%28surgical+mesh+or+biografts%29+for+treatment+of+pelvic+organ+prolapse+or+stress+urinary+incontinence&selectedTitle=1%7E150, Accessed Nov 2014.
Altman D, Vayrynen T, Engh ME, Axelsen S, Falconer C, Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic organ prolapse. N Eng J Med. 2011;364(19):1826–36.
Feiner B, Jelovesk JE, Maher C. Efficacy and safety of transvaginal mesh kits in the treatment of prolapse in the vaginal apex: a systematic review. Br J Obstet Gynecol. 2009;116:15–24.
Sung VW, Rogers RG, Schaffer JI, Balk EM, Uhliq K, Lau J, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112(5):1131–42.
Bjelic-Radisic V, Aigmueller T, Preyer O, Ralph G, Geiss I, Muller G, et al. Vaginal prolapse surgery with transvaginal mesh: results of the Austrian registry. Int Urogynecol J. 2014;25(8):1047–52.
Maher CM, Feiner B, Baessler K, Glazener CM. Surgical management of pelvic organ prolapse in women: the updated summary version cochrane review. Int Urogynecol J. 2011;22(11):1445–57.
El-Khawand D, Wehbe S, O’Hare P, Arunachalam D, Vakhali B. Risk factors for vaginal mesh exposure after mesh-augmented anterior repair: a retrospective cohort. Female Pelvic Med Reconstr Surg. 2014;20(6):305–9.
Food and Drug Administration. FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. Silver spring: FDA; 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm Accessed Nov 2014.
Food and Drug Administration. FDA safety communication: UPDATE on serious complications associated with transvaginal placement of surgical mesh for pelvic organ prolapse. Silver Spring: FDA; 2011. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm262435.htm.
Food and Drug Administration news release. FDA strengthens requirements for surgical mesh for the transvaginal repair of pelvic organ prolapsed to address safety risks. 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm479732.htm, Accessed May 2016.
FDA Executive Summary, Obstetrics and gynecology devices advisory committee meeting, Sept 8–9, 2011. Surgical mesh for treatment of pelvic organ prolapse (POP) and stress urinary incontinence. http://www.fda.gov/downloads/UCM270402.pdf, Accessed Nov 2014.
MRHA, Medicines and Healthcare Products Regulatory Agency. A summary of the evidence on the benefits and risks of vaginal mesh implants. 2014. https://www.gov.uk/government/publications/vaginal-mesh-implants-summary-of-benefits-and-risks, Accessed Nov 2014.
FDA. Urogynecology surgical mesh implants. http://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/. Accessed May 2016.
Haylen BT, Freeman RM, Swift SE, Cosson M, Davila GW, Deprest J, et al. An International Urogynecological Association (IUGA)/ International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22:3–15.
Batelden RP, Weinstein MM, Foust-Wright C, Alperin M, Wakamatsu MM, Pulliam SJ. Clinical application of IUGA/ICS classification system for mesh erosion. Neurourol Urodyn. 2016;35(5):589–94.
AUGS (American Urogynecological Society). Position statement on restriction of surgical options for pelvic floor disorders. 2013. http://www.augs.org/p/bl/et/blogid=6&blogaid=160.pdf file available at this URL. Accessed Nov 2014.
American Urogynecologic Society’s Guidelines Development Committee. Guidelines for providing privileges and credentials to physicians for transvaginal placement of surgical mesh for pelvic organ prolapse. Female Pelvic Med Reconstr Surg. 2012;18(4):194–7.
Clemons JL, Weinstein M, Guess MK, Alperin M, Moalli P, Gregory WT, et al. Impact of the 2011 FDA transvaginal mesh safety update on AUGS members use of synthetic mesh and biologic grafts in pelvic reconstructive surgery. Female Pelvic Med Reconstr Surg. 2013;19(4):191–8.
Reyonlds WS et al. Immediate effects of the initial FDA notification on the use of surgical mesh for pelvic organ prolapse in surgery in medicare beneficiaries. NeurourolUrodyn. 2013;32:330–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Iyer, S., Botros, S.M. Transvaginal mesh: a historical review and update of the current state of affairs in the United States. Int Urogynecol J 28, 527–535 (2017). https://doi.org/10.1007/s00192-016-3092-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-016-3092-7