Abstract
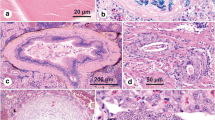
Pearl dace (Semotilus margarita) and brook stickleback (Culaea inconstans) were collected from tributaries of the Athabasca River (Alberta, Canada), upstream (reference site) and downstream of oil sands deposits where fish were expected to be exposed to naturally occurring oil sands constituents. The objective was to determine if fish collected from these sites exhibited differences in the prevalence or intensity of infection by parasites or in gill histology. Dace did not display significant differences in these parameters. Alternately, upstream stickleback were predominantly infected by complex life history parasites, while downstream fish were primarily infected by parasites with simpler life histories. Moreover, downstream stickleback exhibited significantly more clubbing and aneurysms in secondary gill lamellae relative to upstream fish. This suggested a difference in habitat quality between upstream and downstream sites. However, based on basic body condition parameters of the fish, it would appear that any impacts upon the health of the fish due to the presence of naturally occurring oil sands associated chemical constituents would have been minor.

Similar content being viewed by others
References
Arai HP (1989) Guide to the parasites of fishes of Canada. Part III. DFO, Ottawa
Baldwin R, Goater CP (2003) Circulation of parasites among fishes from lakes in the Caribou Mountains, Alberta, Canada. J Parasitol 89:215–225
Bangham RV, Venard CV (1946) Parasites of fish of Algonquin Park lakes. Univ Toronto Stud Biol Ser 53:31–46
Bauer ON (1987) Key to parasites of freshwater fishes of the USSR. Nauka, Leningrad
Blanar CA, Hewitt M, McMaster M et al (2016) Parasite community similarity in Athabasca River trout-perch (Percopsis omiscomaycus) varies with local-scale land use and sediment hydrocarbons, but not distance or linear gradients. Parasitol Res 115:3853–3866
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Canadian Association of Petroleum Producers (2013) Crude oil forecast, markets and transportation. CAPP, Calgary, pp. 1–43
Canadian Canadian Council on Animal Care (CCAC) (1998) CCAC guidelines on: the care and use of fish in research teaching and testing. CCAC, Ottawa, ON, p 85
Carls MG, Rice SD, Hose JE (1999) Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring (Clupea pallasi). Environ Toxicol Chem 18:481–493
Chubb JC (1982) Seasonal occurrence of helminths in freshwater fishes part IV. Adult Cestoda, Nematoda and Acanthocephala. Adv Parasitol 20:1–292
Colavecchia MV, Backus SM, Hodson PV, Parrott JL (2004) Toxicity of oil sands to early life stages of fathead minnows (Pimephales promelas). Environ Toxicol Chem 23:1709–1718
Colavecchia M, Hodson P, Parrott J (2006) CYP1A induction and blue sac disease in early life stages of white suckers (Catostomus commersoni) exposed to oil sands. J Toxicol Environ Health A 69:967–994
Cunningham GR (2006) Pearl dace (Margariscus margarita): a technical conservation assessment. USDA Forest Service, Rocky Mountain Region
Forest JJH (2011) A taxonomic revision of Octomacrum Mueller, 1934 (Monogenea: Polyopisthocotylea: Octomacridae), including an 18 S DNA based phylogeny. Dissertation, Saint Mary’s University, Halifax, Nova Scotia, Canada
Gibson DI (1996) Guide to the parasites of fishes of Canada. Part IV. DFO, Ottawa
Headley JV, Akre C, Conly FM et al (2001) Preliminary characterization and source assessment of PAHs in tributary sediments of the Athabasca River, Canada. Environ Forensics 2:335–345
Hoffman GL (1979) Helminthic parasite. In: Principle diseases of farm-raised catfish, vol 225. Agricultural Experiment Station. Auburn University, Auburn, pp 19–40
Karges RG, Woodward B (1984) Development of lamellar epithelial hyperplasia in gills of pantothenic acid-deficient rainbow trout, Salmo gairdneri Rich. J Fish Biol 25:57–62
Kavanagh RJ, Burnison BK, Frank RA et al (2009) Detecting oil sands process-affected waters in the Alberta oil sands region using synchronous fluorescence spectroscopy. Chemosphere 76:120–126
Kavanagh RJ, Frank RA, Solomon KR, Van Der Kraak G (2013) Reproductive and health assessment of fathead minnows (Pimephales promelas) inhabiting a pond containing oil sands process-affected water. Aquat Toxicol 130–131:201–209
Kaya H, Çelik ES, Gürkan M et al (2013) Effects of subchronic exposure to phosalone on oxidative stress and histopathological alterations in common carp (Cyprinus carpio, L., 1758). J Toxicol Environ Health A 76:853–864
Kelly EN, Schindler DW, Hodson PV et al (2010) Oil sands development contributes elements toxic at low concentrations to the Athabasca River and its tributaries. Proc Nat Acad Sci USA 107:16178–16183
Khan RA, Thulin J (1991) Influence of pollution on parasites of aquatic animals. Adv Parasitol 30:201–238
Lafferty KD, Kuris AM (1999) How environmental stress affects the impacts of parasites. Limnol Oceanogr 44:925–931
Lin YH, Lin HY, Shiau SY (2012) Estimation of dietary pantothenic acid requirement of grouper, Epinephelus malabaricus according to physiological and biochemical parameters. Aquaculture 324–325:92–96
MacKenzie K (1999) Parasites as pollution indicators in marine ecosystems: a proposed early warning system. Mar Pollut Bull 38:955–959
MacKenzie K, Williams HH, Williams B et al (1995) Parasites as indicators of water quality and the potential use of helminth transmission in marine pollution studies. Adv Parasitol 35:85–144
Marty GD, Hose JE, McGurk MD et al (1997) Histopathology and cytogenetic evaluation of Pacific herring larvae exposed to petroleum hydrocarbons in the laboratory or in Prince William Sound, Alaska, after the Exxon Valdez oil spill. Can J Fish Aquat Sci 54:1846–1857
McDonald TE, Margolis L (1995) Synopsis of the parasites of fishes of Canada: Supplement (1978–1993). Canadian Special Publication of Fisheries and Aquatic Sciences 122:1–265
McNeill SA, Arens CJ, Hogan NS et al (2012) Immunological impacts of oil sands-affected waters on rainbow trout evaluated using an in situ exposure. Ecotoxicol Environ Saf 84:254–261
Miller MR, Hinton DE, Stegeman JJ (1989) Cytochrome P-450E induction and localization in gill pillar (endothelial) cells of scup and rainbow trout. Aquat Toxicol 14:307–322
Moore JW, Ramamoorthy S (1984) Aromatic hydrocarbons-polycyclics. In: Organic chemicals in natural waters: applied monitoring and impact assessment. Springer, New York, pp 67–87
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Academia, Praha
Nero V, Farwell, A, Lister, A et al (2006) Gill and liver histopathological changes in yellow perch (Perca flavescens) and goldfish (Carassius auratus) exposed to oil sands process-affected water. Ecotoxicol Environ Saf 63:365–377
Nilsson GE, Dymowska A, Stecyk JAW (2012) New insights into the plasticity of gill structure. Respirat Physiol Neurobiol 184:214–222
Overstreet RM (1993) Parasitic diseases of fishes and their relationship with toxicants and other environmental factors. In: Couch JA, Fournie JW (eds) Pathobiology of marine and estuarine organisms. CRC Press, Boca Raton, pp 111–156
Pietrock M, Marcogliese D, Meinelt T, McLaughlin J (2002) Effects of mercury and chromium upon longevity of Diplostomum sp. (Trematoda: Diplostomidae) cercariae. Parasitol Res 88:225–229
Pietrock M, Meinelt T, Marcogliese DJ (2008) Effects of cadmium exposure on embryogenesis of Stagnicola elodes (Mollusca, Gastropoda): potential consequences for parasite transmission. Arch Environ Contam Toxicol 55:43–48
Regional Aquatic Monitoring Program (RAMP) (2016) Water quality monitoring database. http://www.ramp-alberta.org/data/map/default.aspx?c=Water. Accessed 28 January 2016
Tallman RF, Gee JH (1982) Intraspecific resource partitioning in a headwaters stream fish, the pearl dace Semotilus margarita (Cyprinidae). Env Biol Fish 7:243–249
Tetreault GR, McMaster ME, Dixon DG, Parrott JL (2003) Using reproductive endpoints in small forage fish species to evaluate the effects of Athabasca oil sands activities. Environ Toxicol Chem 22:2775–2782
Thomas EM, Leo M (1995) Synopsis of the parasites of fishes of Canada. NRC Research Press, Ottawa
Timoney KP, Lee P (2009) Does the Alberta tar sands industry pollute? The scientific evidence. Open Conserv Biol J 3:65–81
Tomkins AM, Gee JH (1983) Foraging behavior of brook stickleback, Culaea inconstans (Kirtland): optimization of time, space, and diet. Can J Zool 61:2482–2490
van den Heuvel MR, Power M, Richards J et al (2000) Disease and gill lesions in yellow perch (Perca flavescens) exposed to oil sands mining-associated waters. Ecotoxicol Environ Saf 46:334–341
Wiseman SB, He Y, Gamal-El Din M et al (2013) Transcriptional responses of male fathead minnows exposed to oil sands process-affected water. Comp Biochem Physiol C 157:227–235
Yergeau E, Lawrence JR, Sanschagrin S et al (2012) Next-generation sequencing of microbial communities in the Athabasca River and its tributaries in relation to oil sands mining activities. Appl Environ Microbiol 78:7626–7637
Acknowledgements
The authors gratefully acknowledge technical support by Vijay Tumber and Amber Matthews. Funding provided through PERD, Natural Resources Canada to JLP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raine, J.C., Pietrock, M., Willner, K. et al. Parasitological Analysis and Gill Histopathology of Pearl Dace (Semotilus Margarita) and Brook Stickleback (Culaea Inconstans) Collected from the Athabasca Oil Sands Area (Canada). Bull Environ Contam Toxicol 98, 733–739 (2017). https://doi.org/10.1007/s00128-017-2078-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2078-6