Abstract
Aims/hypothesis
Insulin is a key metabolic regulator in health and diabetes. In pancreatic beta cells, insulin release is regulated by the major second messengers Ca2+ and cAMP: exocytosis is triggered by Ca2+ and mediated by the cAMP/protein kinase A (PKA) signalling pathway. However, the causal link between these two processes in primary beta cells remains undefined.
Methods
Time-resolved confocal imaging of fluorescence resonance energy transfer signals was performed to visualise PKA activity, and combined membrane capacitance recordings were used to monitor insulin secretion from patch-clamped rat beta cells.
Results
Membrane depolarisation-induced Ca2+ influx caused an increase in cytosolic PKA activity via activating a Ca2+-sensitive adenylyl cyclase 8 (ADCY8) subpool. Glucose stimulation triggered coupled Ca2+ oscillations and PKA activation. ADCY8 knockdown significantly reduced the level of depolarisation-evoked PKA activation and impaired replenishment of the readily releasable vesicle pool. Pharmacological inhibition of PKA by two inhibitors reduced depolarisation-induced PKA activation to a similar extent and reduced the capacity for sustained vesicle exocytosis and insulin release.
Conclusions/interpretation
Our findings suggest that depolarisation-induced Ca2+ influx plays dual roles in regulating exocytosis in rat pancreatic beta cells by triggering vesicle fusion and replenishing the vesicle pool to support sustained insulin release. Therefore, Ca2+ influx may be important for glucose-stimulated insulin secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin regulates blood glucose metabolism and helps maintain energy homeostasis. Impaired insulin secretion leads to glucose intolerance and diabetes. In response to increased blood glucose, the cytosolic ATP/ADP ratio in pancreatic beta cells increases [1]. This change leads to the closure of KATP channels, which depolarises the membrane, causing Ca2+ influx and subsequent exocytosis of insulin granules [2, 3]. Thus, elevated intracellular Ca2+ ([Ca2+]i) in pancreatic beta cells is known to be the major trigger for glucose-stimulated insulin secretion.
Cyclic AMP (cAMP) is another important second messenger that enhances vesicle fusion via both protein kinase A (PKA)-dependent and PKA-independent pathways, which are dependent on exchange protein directly activated by cAMP 2 (EPAC2) [4–6]. Endogenous incretin hormones, such as glucose-dependent insulinotropic polypeptide [7] and glucagon-like peptide1 (GLP-1) [8], act on membrane receptors to generate cytoplasmic cAMP and regulate insulin secretion. Glucose metabolism also triggers cytoplasmic cAMP elevation and oscillations in pancreatic beta cells, although the underlying mechanisms and the physiological significance of this process remain unclear [9–12].
Crosstalk between these key regulators of insulin secretion, the Ca2+ and cAMP signalling pathways, occurs within individual beta cells. Both Ca2+-activated and Ca2+-inhibited adenyl cyclase (ADCY) isoforms are expressed in insulin-secreting cells [13]. Ca2+-activated phosphodiesterase (PDE) has been proposed to mediate cAMP oscillations in clonal MIN6 cells [14, 15]. Similarly, cAMP accumulation activates PKA, leading to phosphorylation of voltage-gated Ca2+ channels (VGCCs) on the plasma membrane [16, 17] and inositol 1,4,5-trisphosphate and ryanodine receptors on the endoplasmic membrane [18, 19]. Owing to the complexity of these interactions, the temporal and causal relationships between the Ca2+- and cAMP/PKA-mediated pathways in beta cells remain undefined [11, 13–15, 20, 21]. It is unclear whether and how glucose or depolarisation triggers activation of the cAMP/PKA pathway, and vice versa.
In the present study, we used a genetically encoded AKAR3 reporter [22] to monitor the real-time activity of PKA in primary beta cells using fluorescence resonance energy transfer (FRET). By combining membrane capacitance (Cm) measurements with FRET imaging in individual beta cells, we showed that depolarisation-induced Ca2+ influx activates PKA via an adenylyl cyclase 8 (ADCY8) subpool and thus helps to replenish the readily releasable pool (RRP) of insulin granules during glucose stimulation.
Methods
Detailed materials and methods are available in the electronic supplementary materials (ESM Methods).
Cell culture and transfection
Pancreatic islet beta cells from adult Wistar rats (150–250 g) were isolated and cultured as previously described [23]. Briefly, rats were killed by cervical dislocation and islets were obtained from the pancreas by collagenase P digestion. After short-term tissue culture (4–24 h) in RPMI 1640 medium, single beta cells were isolated by treating the islets with 0.025% trypsin (Invitrogen, Carlsbad, CA, USA). Transfection was conducted using the Neon 10 μl transfection system MPK10096 (Invitrogen). Experiments were performed 24–72 h after transfection, or >72 h for ADCY8 knockdown (KD).
Real-time FRET and Ca2+ imaging
Live imaging of the AKAR3 fluorescent PKA reporter was performed using a Zeiss 710 inverted confocal microscope (Carl Zeiss, Oberkochen, Germany). AKAR3 was excited using a 405 nm laser, and a simultaneous two-channel mode was used for emission detection: one channel for cyan (466–489 nm) and the other for yellow (519–535 nm). The FRET ratio was calculated as R = FYFP/FCFP, and normalised as ΔR = ΔR′/R, where ΔR′ is the absolute change in the FRET ratio, to take into account variation in the basal FRET ratio among different cells. For simultaneous imaging of [Ca2+]i and FRET, cells were preloaded with 5 μmol/l Rhod-2 AM (Invitrogen) for <10 min, and the switched mode of frame-scan was used to alternately detect FRET and Ca2+ signals. Ca2+ signals were detected at 543 nm excitation and 560–620 nm emission. Images were acquired at 0.5 Hz, and the temperature was maintained at 28–32°C using a TempModule S (Carl Zeiss). Control solutions and drugs were applied locally to individual cells during recording using a multichannel microperfusion system [24].
Electrophysiology and membrane capacitance recording
Whole-cell and perforated whole-cell configurations were used as previously described [3, 25]; the latter configuration was used during imaging to avoid loss of fluorescence intensity resulting from protein leakage into the pipette. For H-89 inhibition experiments, cells were dialysed with a solution containing 15 μmol/l H-89 for >7 min. Beta cells were characterised as healthy cells with a Cm of >4 pF and no Na+ current [23]. The standard extracellular solution contained 118 mmol/l NaCl, 20 mmol/l tetraethylammonium chloride (TEA), 5.6 mmol/l KCl, 2.6 mmol/l CaCl2·2H2O, 1.2 mmol/l MgCl2, 5 mmol/l d-glucose and 5 mmol/l HEPES at pH 7.4. The intracellular solution contained 152 mmol/l CsCH3SO3, 10 mmol/l CsCl, 10 mmol/l KCl, 1 mmol/l MgCl2 and 5 mmol/l HEPES, with pH adjusted to 7.35 using CsOH.
Detection of insulin release
Insulin release from beta cells was measured by ELISA as previously described [25]. For ELISA, we used Krebs-Ringer buffer (KRB; 5 mmol/l KCl, 120 mmol/l NaCl, 15 mmol/l HEPES, pH 7.4, 24 mmol/l NaHCO3, 1 mmol/l MgCl2, 2 mmol/l CaCl2 and 1 mg/ml BSA). Cells were treated with 105 mmol/l KCl for 1 min and the incubation solution was then collected for analysis. After incubation for 10 min with KRB (vehicle), PKA blocker (H-89) or ADCY8 blocker (2′,5′-dideoxyadenosine [DDA]), cells were treated with KCl for a further 1 min. All samples were then centrifuged at 16,000 g for 5 min, and insulin levels in the supernatants were determined.
Statistical analysis
All data were collected and analysed using Igor software (WaveMetrix, Lake Oswego, OR, USA). The means ± SEM were calculated, and the Student’s t test was used to compare treatment effects. Statistical significance was set at p < 0.05.
Results
Depolarisation induces PKA activation in pancreatic beta cells
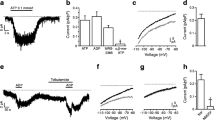
AKAR3, a genetically encoded fluorescent PKA reporter, was used to visualise PKA activity in beta cells. AKAR3 is a fusion peptide comprising cyan fluorescent protein (CFP), a phosphoamino acid-binding domain (14-3-3τ), a PKA-specific phosphorylatable peptide and yellow fluorescent protein (YFP). 14-3-3τ mediates signal transduction by binding to a PKA-phosphorylated substrate peptide. The resulting intramolecular conformational change alters the distance between CFP and YFP, thus leading to a reversible FRET signal [22]. As expected, when AKAR3 was transfected into primary beta cells, fluorescence was homogeneously distributed within the cytoplasm (Fig. 1e). The addition of 100 μmol/l 3-isobutyl-1-methylxanthine (IBMX; a PDE inhibitor) increased PKA activity, as shown by an increase in ΔR (Fig. 1a, d; n = 13). Therefore, the AKAR3 probe can detect changes in PKA activity in live primary beta cells.
FRET imaging of depolarisation-induced PKA activation in primary beta cells. (a, b) Changes in CFP (cyan) and YFP (yellow) signal intensity and ΔR (black) during the application of 100 μmol/l IBMX or 105 mmol/l KCl for 25 s to pancreatic beta cells. (c) ΔR changes during the application of 100 μmol/l IBMX or 105 mmol/l KCl for 100 s to the same cells (n = 10). (d) Summary of ΔR changes, as measured in (a–c). (e) Experimental set-up and representative patch-clamp recording of AKAR3-expressing beta cells. Changes in CFP and YFP intensity and ΔR during a single 500 ms depolarisation event from −70 mV to 0 mV are also shown. (f, g) ΔR amplitude and half duration of FRET signals, as described in (e) (n = 8 out of 32 responsive cells). Dep., depolarisation; SE, standard extracellular solution
Stimulation of cells with 105 mmol/l KCl for 25 s (n = 14) also evoked a robust increase in ΔR, comparable with that evoked by IBMX, followed by a slower (1–2 min) return to baseline (Fig. 1b, d). This ΔR value could not have been affected by intracellular pH because the pH did not alter during KCl treatment (ESM Fig. 1). A longer depolarisation period (100 s, n = 10) did not lead to a further increase in ΔR (Fig. 1c, d), but was associated with a longer recovery time (time constant, tau) to the basal level (tau = 171 ± 45 s). In contrast, a longer application of IBMX (n = 10) induced a much greater increase in ΔR (0.42 ± 0.05), but with a similar recovery time (tau = 54 ± 9 s) as that following a 25 s IBMX treatment (Fig. 1c, d). Therefore, membrane depolarisation-induced PKA activity is saturable and reaches its peak value after 25 s KCl stimulation.
Rather than generating persistent membrane depolarisation, as seen with KCl, glucose induces short trains of action potential spikes in beta cells [15, 26]. Therefore, we used a perforated whole-cell voltage clamp, which keeps most of the intracellular metabolites intact during recording [3], to measure PKA activity evoked by a single depolarisation event. A 500 ms single-step depolarisation from −70 to 0 mV, which is comparable with glucose-induced depolarisation, increased PKA activity in 25% of recorded beta cells (Fig. 1e, f; ΔR = 0.08 ± 0.01, n = 8 out of 32 recorded cells). Although the ΔR increase was smaller, the recovery rate (tau = 94 ± 19 s) after depolarisation was similar to that induced by 25 s KCl stimulation (Fig. 1e, f). Thus, physiologically relevant depolarisation conditions can activate PKA in a proportion of beta cells.
Depolarisation-induced PKA activation is Ca2+ dependent
To directly assess the temporal relationship between a depolarisation-evoked increase in [Ca2+]i and PKA activation, we preloaded AKAR3-transfected beta cells with the Ca2+ indicator, Rhod-2 AM. The [Ca2+]i increased rapidly following the application of 105 mmol/l KCl and then rapidly decreased to the basal level (Fig. 2c–e, rise time (RT) = 27 ± 2 s; tau = 63 ± 15 s, n = 11). These results contrast with the much slower changes in PKA activity (Fig. 2a, c; RT = 59 ± 5 s; tau = 116 ± 24 s, n = 7). Latency between the maximum [Ca2+]i and ΔR peaks was 28 ± 5 s, indicating that PKA activation lags [Ca2+]i elevation (Fig. 2f).
Depolarisation-induced PKA activation is Ca2+-dependent in primary beta cells. (a) Depolarisation-induced PKA changes in single beta cells suspended in 2.6 mmol/l Ca2+ or 0 mmol/l Ca2+ containing 2 mmol/l EGTA (n = 7). (b) Graph showing PKA signals, as measured in (a). (c–f) Simultaneous recordings of FRET and [Ca2+]i in single beta cells (n = 11). [Ca2+]i was measured using Rhod-2 AM. RT, rise time; HD, half-height duration; Latency (Ca-to-PKA), latency between [Ca2+]i and PKA signal peaks; **p < 0.01; ***p < 0.001
This temporal association suggests a causal relationship between increased [Ca2+]i and PKA activation. To test this possibility, cells were sequentially stimulated with a KCl solution containing 2.6 mmol/l or 0 mmol/l Ca2+ for 25 s. When cells were depolarised in the Ca2+-free solution, there was no PKA activation (Fig. 2a, b; n = 7); however, the addition of 2.6 mmol/l Ca2+ partially restored PKA activation. Therefore, depolarisation-induced PKA activation is Ca2+ dependent.
When isolated single cells were challenged with 20 mmol/l glucose, oscillatory changes in PKA activity occurred at a frequency of approximately 0.3/min (Fig. 3a; n = 8). Simultaneous monitoring of [Ca2+]i and PKA activity showed a good temporal association between [Ca2+]i spikes and ΔR spikes (Fig. 3b), implying a link between Ca2+ influx and PKA activation in glucose-mediated physiological processes, including glucose-stimulated insulin secretion.
Glucose-induced PKA oscillations are coupled to [Ca2+]i oscillations in beta cells. (a) PKA oscillations induced by 20 mmol/l glucose (n = 8). (b) Simultaneous recording of oscillatory [Ca]i (ΔF, red) and PKA (ΔR, black) signals induced by 20 mmol/l glucose in a representative cell bathed in standard extracellular solution (SE) (n = 5)
ADCY8 is required for Ca2+-dependent PKA activation
Ca2+ modulates the intracellular free cAMP concentration ([cAMP]i) and PKA activity via either Ca2+-sensitive ADCY or Ca2+-sensitive PDE [27]. Because depolarisation/Ca2+-induced PKA activation showed different kinetics (slower decay rate) from those evoked by PDE inhibition (Fig. 1a, b), we speculated that ADCYs may play an important role. ADCY8 is activated by Ca2+/calmodulin and participates in glucose- and GLP-1-mediated cAMP production in pancreatic beta cells [13, 28]. We therefore used RNA interference (RNAi)-based ADCY8 KD to determine the role of ADCY8 in depolarisation-evoked PKA activation.
ADCY8 was efficiently silenced by ADCY8 short hairpin RNA (sh-ADCY8) transfection; a scrambled short hairpin RNA served as control (sh-Ctr). This effect was completely reversed by expression of a short hairpin RNA-resistant form of ADCY8 (‘rescue’) in INS-1 cells (Fig. 4a, b; n = 3). Membrane depolarisation with 105 mmol/l KCl induced a robust ΔR increase in sh-Ctr-expressing cells (Fig. 4c, f; ΔR = 0.08 ± 0.02, n = 20), but failed to activate PKA in most ADCY8-KD cells (Fig. 4d, f; ΔR = 0.017 ± 0.014, n = 18). Treatment with 100 μmol/l IBMX (positive control) evoked similar ΔR changes in both ADCY8 KD and control cells (Fig. 4c, d). Depolarisation-evoked PKA activation was probably caused by reduced ADCY8 expression because it was completely rescued by ADCY8 overexpression (Fig. 4e, f; ΔR = 0.17 ± 0.04, n = 5). Therefore, ADCY8 mediates depolarisation-evoked PKA activation in rat primary beta cells.
ADCY8 is required for Ca2+-dependent PKA activation in pancreatic beta cells. (a, b) Sh-ADCY8 KD and rescue efficiency in INS-1 cells, as assessed by RT-PCR and real-time PCR (n = 3). (c–e) PKA changes induced by high KCl and IBMX in control (sh-Ctr; n = 20), ADCY8-KD (sh-ADCY8; n = 18) and rescued (sh-ADCY8 + rescue; n = 5) beta cells. (f) Graphs summarising results from (c–e). p < 0.05; ** p < 0.01
Depolarisation-induced PKA activation replenishes depleted vesicle pools
To investigate the role of PKA in vesicle pool replenishment, we stimulated primary beta cells with depolarising pulse (or stimulus) trains from −70 to 0 mV at 100 ms intervals, and simultaneously measured ΔR (i.e. PKA activity) and Cm (i.e. exocytosis) in single beta cells (Fig. 5a). Each pulse train comprised five 50 ms depolarising pulses to deplete the immediately releasable pool (IRP1), followed by eight 500 ms depolarising pulses to induce exocytosis from the readily releasable pool (RRP1) [29]. A second identical stimulus pulse train was repeated 2 min after the first pulse train (IRP2 and RRP2, respectively). Recovery was estimated by determining the IRP2/IRP1 and RRP2/RRP1 ratios in the same cells.
Depolarisation-induced PKA activation is responsible for vesicle pool replenishment. (a) Representation showing simultaneous recording of PKA activity (ΔR) and Cm (exocytosis). (b) A train of depolarising pulses from −70 mV to 0 mV using voltage clamp-induced PKA (above) and exocytosis (below) signals in a single beta cell using a perforated whole-cell configuration. The stimulus train consisted of five 50-ms pulses to deplete vesicles from the IRP followed by eight 500-ms pulses to elicit exocytosis from the RRP, with 100 ms intervals between pulses. The stimulus trains were repeated twice, with a 2 min intervals between the first (Cm1, black) and second (Cm2, blue) stimulus train to allow vesicle pool replenishment. (c) Similar to (b) except that 15 μmol/l H-89 (PKA inhibitor) was present in the patch pipette. (d, e) Graphs showing Cm2/Cm1 ratios for IRP and RRP (d) and PKA (e) evoked by the first pulse train in control (n = 5) and H-89 treated (n = 6) cells. (f) PKA activation is responsible for sustained insulin release from beta cells. Insulin release was stimulated by two pulses of KCl (105 mmol/l, 1 min; 10 min interval) and measured by ELISA. H-89 (15 μmol/l) or DDA (100 μmol/l) significantly inhibited insulin release following the second stimulus to 0.42 (p < 0.01) and 0.25 (p < 0.01) ng/ml, respectively (n = 4). p < 0.05; ** p < 0.01; ***p < 0.001
Typically, the first depolarising pulse train led to an increase in ΔR comparable to that evoked by a 100 s KCl stimulation (Fig. 5b, e; ΔR = 0.20 ± 0.06, n = 5). The second pulse train was applied approximately 100 s later, when the cytoplasmic PKA activity had returned to the basal level (Fig. 5b). Under control conditions, Cm changes triggered by the second pulse train were similar to those triggered by the first (Fig. 5b, d; IRP2/IRP1 1.08 ± 0.07, RRP2/RRP1 0.95 ± 0.05), indicating complete replenishment of both pools (IRP and RRP). In contrast, 7–10 min after cell dialysis with a patch pipette containing H-89 (a membrane-permeable PKA inhibitor [30]), no increase in ΔR was induced by the first or second depolarising pulse train (Fig. 5c, e), indicating a complete blockade of PKA activation by H-89. The IRP (75 ± 12 fF) and RRP (279 ± 18 fF) induced by the first depolarising pulse train remained unchanged in the presence of H-89. However, the recovery of these pools before the second pulse train was severely impaired by PKA inhibition (Fig. 5c, d; IRP2/IRP1 0.71 ± 0.06, RRP2/RRP1 0.43 ± 0.08, n = 6).
Next, we examined insulin release from dispersed islet beta cells using ELISA. Two sequential depolarisation events 10 min apart each triggered almost the same amount of insulin release in control cells (Fig. 5f). However, cells treated with either H-89 or the transmembrane ADCY inhibitor DDA during the interval between depolarising pulse trains exhibited significantly less insulin release in response to the second vs the first stimulus (Fig. 5f; n = 4 for each experimental condition). Consistent with this, Rp-cAMPS, a more selective cAMP/PKA inhibitor, had similar inhibitory effects on both phases of glucose-induced insulin secretion (ESM Fig. 2). Taken together, these findings suggest that PKA activity stimulated by depolarisation and/or Ca2+ entry plays a central role in refilling insulin vesicle pools in rat primary beta cells.
ADCY8 activation facilitates sustained vesicle fusion
Similar levels of PKA activation were induced by either a standard depolarising pulse train lasting for 5 s or a 100 s KCl stimulation (Figs 1d and 5e), suggesting that Ca2+ influx evoked by a 5 s pulse train maximally activates ADCY8 and PKA upon membrane depolarisation. In addition to Ca2+, GLP-1 is known to activate ADCY8 via stimulation of the GLP-1 receptor [28]. In primary beta cells, 100 nmol/l GLP-1 evoked a much greater level of PKA activation compared with KCl (ESM Fig. 3; n = 5). GLP-1-induced PKA activity was reduced by approximately 60% in ADCY8-KD cells (ESM Fig. 4), suggesting that ADCY8 also has a major role in hormone-induced cAMP/PKA activation. In addition, ADCY8 KD had a greater effect on GLP-1-triggered PKA activation (approximately 60% reduction; ESM Fig. 4) than on PKA activation evoked by membrane depolarisation (Fig. 4f), indicating that membrane depolarisation does not activate all of the functional ADCY8 in beta cells. To confirm that the Ca2+-responsive ADCY8 subpool is functionally important, we used a standard whole-cell patch-clamp configuration with an ATP- and cAMP-free intracellular solution to examine the role of ADCY8 in vesicle pool replenishment. In sh-Ctr-expressing cells, the increase in Cm induced by the second depolarising pulse train was somewhat lower than that of the first pulse train (by 33%) as a result of rundown (Fig. 6a, c; IRP2/IRP1 0.97 ± 0.19, RRP2/RRP1 0.67 ± 0.07; but see [5]). Thus, in control cells, the first pulse train evoked transient PKA activation that causes refilling of most of the IRP and RRP. In ADCY8-KD cells, the depolarisation-induced [Ca2+]i rise was intact (ESM Fig. 5; n = 11) and the first pulse train induced a Cm increase similar to that of control cells (Fig. 6b, c; IRP 15.0 ± 2.8 fF, RRP 85.6 ± 17.1 fF); however, secretion induced by the second pulse train was dramatically impaired (Fig. 6b, d; IRP2/IRP1 0.42 ± 0.12, RRP2/RRP1 0.35 ± 0.09). The recovery rate was markedly slower in ADCY8-KD cells than in control cells (Fig. 6a, d), indicating that replenishment of vesicle pools is impaired by ADCY8 KD. Taken together, these data suggest that activation of ADCY8 by depolarisation is responsible for replenishing insulin vesicle pools in rat beta cells.
ADCY8 is responsible for vesicle pool replenishment. (a, b) Cm recordings under whole-cell dialysis with an ATP- and cAMP-free solution in ADCY8 KD (n = 11) and control (n = 6) cells. (c, d) Graphs summarising exocytosis and Cm2/Cm1 ratios for both IRP and RRP induced by the first pulse train, from (a, b). p < 0.05
Discussion
A major finding of the present study is that depolarisation-induced Ca2+ influx has dual effects on insulin release in beta cells, by directly triggering vesicle fusion (for instant release) and indirectly promoting vesicle replenishment (for sustained release). Regarding the relationship between Ca2+ and vesicle pools, it has been established that intracellular Ca2+ triggers vesicle release via stimulating Ca2+-dependent vesicle fusion to the plasma membrane in neurons and endocrine cells [24, 31–33], including beta cells [25, 34–36]. However, multiple mechanisms have been proposed to promote vesicle pool replenishment in excitable cells. In chromaffin cells and neurons, it is known that basal [Ca2+]i level can modulate the vesicle pool [37, 38]. Previous pharmacological experiments established that cAMP also facilitates replenishment of the vesicle pool in beta cells via both PKA-dependent and EPAC2-dependent pathways [2, 4–6, 34]. Here, by combining PKA imaging with Cm recording, we found that ADCY8 links the Ca2+- and cAMP/PKA-dependent pathways (described above) in rat primary beta cells. When using a physiologically relevant stimulation pattern (Fig. 3), the first depolarising pulse train caused not only the first phase of secretion and PKA activation but also facilitated exocytosis/insulin release induced by the second pulse train through vesicle pool replenishment supported by PKA activation (Figs 5 and 6). These findings suggest that Ca2+- and ADCY8-dependent PKA pathways may also play critical roles in promoting sustained insulin release upon glucose challenge under physiological conditions.
The second important finding is the causal link between depolarisation-induced Ca2+ entry and enhanced PKA activity in rat primary beta cells. Previous studies using genetic indicators of cAMP levels in clonal MIN6 cells showed coordinated glucose-stimulated oscillations in Ca2+ and cAMP [14, 20], as well as Ca2+ influx and an ADCY8-induced cAMP signal [39]. A recent study reported that Ca2+ and cAMP oscillate in synchrony and that PKA activity lags behind cAMP changes such that an increase in PKA activity precedes the next round of Ca2+ signalling after depolarisation in MIN6 cells [15]. Differences between stimuli (KCl/glucose vs TEA) may account for the observed differences in lag times between different signals. In the present study, we demonstrated that a physiologically relevant depolarisation-triggered transient increase in [Ca2+]i precedes the transient PKA activation by 28 s in primary beta cells (Fig. 2c). Moreover, the depolarisation-evoked PKA activation was abolished by removing extracellular Ca2+ (Fig. 2a, b). Glucose-stimulated PKA activity was also coupled to [Ca2+]i, although the lag time and the waveform linking them were variable (Fig. 3b). This variation may be caused by glucose promotion of cAMP production via metabolic coupling factors unrelated to Ca2+ [11, 20, 40]. These results established the important contribution of elevated [Ca2+]i to activation of the glucose-triggered cAMP/PKA pathway in native beta cells, as distinct from previous studies using cell lines.
Our third finding is that an ADCY8 subpool in beta cell contributes to depolarisation-induced PKA activation. ADCY8 is an important mediator of cAMP accumulation evoked by incretin stimulation [28]. Previous studies also revealed that A kinase-anchoring protein 79/150 (AKAP79/150) specifically interacts with ADCY8 and thus has the potential to produce localised cAMP signalling evoked by local Ca2+ entry [41]. Here, we showed that the level of PKA activation following depolarisation triggered by 105 mmol/l KCl perfusion for 25–100 s was similar to that triggered by patch-clamp depolarisation for ≤5 s (Figs 1 and 5). This was not a consequence of probe saturation because 100 s IBMX evoked an even greater response in the same cells (Fig. 1c, d). Instead, our interpretation of the data is that an ADCY8 subpool is activated by membrane depolarisation. Although ADCY8 is almost completely absent from human beta cells [12, 42], this finding may help to reveal the roles of other ADCY isoforms, such as ADCY5, which is involved in human islet function [12].
In a physiological context, each wave within the glucose-induced membrane potential oscillations may maximally and repeatedly activate a proportion of the cAMP/PKA pathway. As shown in Fig. 3, PKA peaks triggered by 20 mmol/l glucose had a similar amplitude and dynamics to those evoked by a single train of depolarisation. Such an arrangement ensures sustained insulin vesicle trafficking and secretion in single primary beta cells. Here, consistent with previous reports [13, 40, 43], GLP-1 evoked a much greater level of PKA activation than that evoked by depolarisation alone (ESM Fig. 3), suggesting that incretins activate an additional ADCY8 subpool [43]. ADCY8 proteins activated at later time points may be more distant from VGCCs and thus incapable of activation by the depolarisation-induced Ca2+ influx alone (Fig. 7). Figure 7 summarises the results of present study: glucose stimulation generates a transient microdomain of high [Ca2+]i that promotes insulin secretion through dual pathways, i.e. by directly triggering vesicle fusion and indirectly activating ADCY8/PKA to enhance vesicle pool replenishment. A VGCC-coupled ADCY8 subpool plays a central role in Ca2+-evoked PKA activation, which is partially responsible for glucose- and GLP-1-stimulated cAMP production. This study has improved our understanding of glucose- and Ca2+-regulated vesicle trafficking and pulsatile insulin secretion [2, 3, 44] under normal physiological and diabetic conditions.
Proposed model for the control of insulin release by the glucose/Ca2+/ADCY8 pathway. (a) Following an increase in blood glucose (1), the GLUT delivers glucose into beta cells (2), followed by a mitochondria-mediated increase in the cytosolic ATP/ADP ratio (3). This change leads to KATP channel closure (4) and action potential firing (5). During membrane depolarisation, VGCCs open (6), leading to increased [Ca2+]i (7), which triggers insulin vesicle exocytosis (8a). Increased [Ca2+]i also stimulates ADCY8 activation (8), leading to cAMP production (9) and PKA activation (10). PKA activation is responsible for vesicle pool replenishment (11) and maintaining insulin secretion (12). Incretins (such as GLP-1) activate the additional VGCC-insensitive ADCY8 subpool and can trigger higher levels of cAMP/PKA pathway activation compared with depolarisation. (b) PKA activation stimulates reserve pool vesicles into the RRP necessary for sustained insulin secretion. Mito, mitochondrion
Abbreviations
- ΔR:
-
Normalised FRET ratio
- ADCY:
-
Adenyl cyclase
- [Ca2+]i :
-
Intracellular free Ca2+ concentration
- cAMP:
-
Cyclic AMP
- [cAMP]i :
-
Intracellular free cyclic AMP concentration
- CFP:
-
Cyan fluorescent protein
- Cm :
-
Membrane capacitance
- DDA:
-
2′,5′-dideoxyadenosine
- EPAC2:
-
Exchange protein directly activated by cAMP 2
- FRET:
-
Fluorescence resonance energy transfer
- GLP-1:
-
Glucagon-like peptide1
- IBMX:
-
3-Isobutyl-1-methylxanthine
- IRP:
-
Immediately releasable pool
- KD:
-
Knockdown
- PDE:
-
Phosphodiesterase
- PKA:
-
Protein kinase A
- RRP:
-
Readily releasable pool
- sh-ADCY8:
-
ADCY8 short hairpin RNA
- sh-Ctr:
-
Scrambled control short hairpin RNA
- TEA:
-
Tetraethylammonium chloride
- YFP:
-
Yellow fluorescent protein
- VGCC:
-
Voltage-gated Ca2+ channel
References
Tarasov AI, Semplici F, Ravier MA et al (2012) The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic beta-cells. Plos One 7:e39722
Ashcroft FM, Rorsman P (2012) Diabetes mellitus and the beta cell: the last ten years. Cell 148:1160–1171
Zhou Z, Misler S (1996) Amperometric detection of quantal secretion from patch-clamped rat pancreatic beta-cells. J Biol Chem 271:270–277
Renstrom E, Eliasson L, Rorsman P (1997) Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B cells. J Physiol 502(Pt 1):105–118
Eliasson L, Ma X, Renstrom E et al (2003) SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B cells. J Gen Physiol 121:181–197
Shibasaki T, Takahashi H, Miki T et al (2007) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci U S A 104:19333–19338
Gremlich S, Porret A, Hani EH et al (1995) Cloning, functional expression, and chromosomal localization of the human pancreatic islet glucose-dependent insulinotropic polypeptide receptor. Diabetes 44:1202–1208
Thorens B (1992) Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci U S A 89:8641–8645
Grill V, Cerasi E (1973) Activation by glucose of adenyl cyclase in pancreatic islets of the rat. FEBS Lett 33:311–314
Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR (2008) Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol 132:329–338
Dyachok O, Idevall-Hagren O, Sagetorp J et al (2008) Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab 8:26–37
Hodson DJ, Mitchell RK, Marselli L et al (2014) ADCY5 couples glucose to insulin secretion in human islets. Diabetes 63:3009–3021
Delmeire D, Flamez D, Hinke SA, Cali JJ, Pipeleers D, Schuit F (2003) Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia 46:1383–1393
Landa LR Jr, Harbeck M, Kaihara K et al (2005) Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem 280:31294–31302
Ni Q, Ganesan A, Aye-Han NN et al (2011) Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol 7:34–40
De Marinis YZ, Salehi A, Ward CE et al (2010) GLP-1 inhibits and adrenaline stimulates glucagon release by differential modulation of N- and L-type Ca2+ channel-dependent exocytosis. Cell Metab 11:543–553
Kamp TJ, Hell JW (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87:1095–1102
Kang G, Chepurny OG, Rindler MJ et al (2005) A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol 566:173–188
Dyachok O, Gylfe E (2004) Ca(2+)-induced Ca(2+) release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic beta-cells. J Biol Chem 279:45455–45461
Idevall-Hagren O, Barg S, Gylfe E, Tengholm A (2010) cAMP mediators of pulsatile insulin secretion from glucose-stimulated single beta-cells. J Biol Chem 285:23007–23018
Kim JW, Roberts CD, Berg SA, Caicedo A, Roper SD, Chaudhari N (2008) Imaging cyclic AMP changes in pancreatic islets of transgenic reporter mice. PLoS One 3:e2127
Zhang J, Ma Y, Taylor SS, Tsien RY (2001) Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A 98:14997–15002
Lou XL, Yu X, Chen XK et al (2003) Na+ channel inactivation: a comparative study between pancreatic islet beta-cells and adrenal chromaffin cells in rat. J Physiol 548:191–202
Zhang C, Zhou Z (2002) Ca(2+)-independent but voltage-dependent secretion in mammalian dorsal root ganglion neurons. Nat Neurosci 5:425–430
Dou HQ, Xu YF, Sun JP et al (2012) Thiopental-induced insulin secretion via activation of IP3-sensitive calcium stores in rat pancreatic beta-cells. Am J Physiol Cell Physiol 302:C796–C803
Rorsman P (1997) The pancreatic beta-cell as a fuel sensor: an electrophysiologist’s viewpoint. Diabetologia 40:487–495
Willoughby D, Cooper DM (2007) Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87:965–1010
Roger B, Papin J, Vacher P et al (2011) Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia 54:390–402
Merrins MJ, Stuenkel EL (2008) Kinetics of Rab27a-dependent actions on vesicle docking and priming in pancreatic beta-cells. J Physiol 586:5367–5381
Zhang X, Candas M, Griko NB, Taussig R, Bulla LA Jr (2006) A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc Natl Acad Sci U S A 103:9897–9902
Katz B (1969) The release of neural transmitter substances. Liverpool University Press, Liverpool
Neher E, Sakaba T (2008) Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59:861–872
Sudhof TC (2004) The synaptic vesicle cycle. Annu Rev Neurosci 27:509–547
Ammala C, Ashcroft FM, Rorsman P (1993) Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature 363:356–358
Gustavsson N, Lao Y, Maximov A et al (2008) Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A 105:3992–3997
Michael DJ, Ritzel RA, Haataja L, Chow RH (2006) Pancreatic beta-cells secrete insulin in fast- and slow-release forms. Diabetes 55:600–607
von Ruden L, Neher E (1993) A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science 262:1061–1065
Zhang B, Sun L, Yang YM et al (2011) Action potential bursts enhance transmitter release at a giant central synapse. J Physiol 589:2213–2227
Everett KL, Cooper DM (2013) An improved targeted cAMP sensor to study the regulation of adenylyl cyclase 8 by Ca2+ entry through voltage-gated channels. PLoS One 8:e75942
Tian G, Sandler S, Gylfe E, Tengholm A (2011) Glucose- and hormone-induced cAMP oscillations in alpha- and beta-cells within intact pancreatic islets. Diabetes 60:1535–1543
Willoughby D, Masada N, Wachten S et al (2010) AKAP79/150 interacts with AC8 and regulates Ca2+-dependent cAMP synthesis in pancreatic and neuronal systems. J Biol Chem 285:20328–20342
Leech CA, Castonguay MA, Habener JF (1999) Expression of adenylyl cyclase subtypes in pancreatic beta-cells. Biochem Biophys Res Commun 254:703–706
Fonseca SG, Urano F, Weir GC, Gromada J, Burcin M (2012) Wolfram syndrome 1 and adenylyl cyclase 8 interact at the plasma membrane to regulate insulin production and secretion. Nat Cell Biol 14:1105–1112
Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H (2002) Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science 297:1349–1352
Acknowledgements
We thank H. Ma (Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, China), T. Xie (College of Engineering, Peking University, China) and S. Chen (College of Engineering, Peking University, China) for training in experimental techniques, I. Bruce (Institute of Molecular Medicine, Peking University, China) for his comments on the manuscript.
Funding
This work was supported by grants from the National Basic Research Programme of China (2012CB518006), the National Natural Science Foundation of China (31228010, 31171026, 31100597, 31327901, 81222020, 31221002, 31330024 and 31400708) and the National Key Technology R&D Programme (SQ2011SF11B01041). Changhe Wang was supported in part by the Postdoctoral Fellowship of Peking-Tsinghua Center for Life Science.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
HD, CW, LC, XY and ZZ were responsible for conception and design of the study. HD, CW, XW, LY, XZ, ST, HX, BL, QW, QZ, MH, YW, LW, QL, JS, YW, SS, XK and LZ contributed to data acquisition, analysis and interpretation. MR, JL and JZ contributed to data analysis and interpretation. All authors were involved in drafting the manuscript and all approved the final version. ZZ is the guarantor of this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Haiqiang Dou and Changhe Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 164 kb)
ESM Fig. 1
(PDF 198 kb)
ESM Fig. 2
(PDF 40 kb)
ESM Fig. 3
(PDF 88 kb)
ESM Fig. 4
(PDF 102 kb)
ESM Fig. 5
(PDF 97 kb)
Rights and permissions
About this article
Cite this article
Dou, H., Wang, C., Wu, X. et al. Calcium influx activates adenylyl cyclase 8 for sustained insulin secretion in rat pancreatic beta cells. Diabetologia 58, 324–333 (2015). https://doi.org/10.1007/s00125-014-3437-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3437-z