Abstract
Aims/hypothesis
FTO harbours the strongest known obesity-susceptibility locus in Europeans. While there is growing evidence for a role for FTO in obesity risk in Asians, its association with type 2 diabetes, independently of BMI, remains inconsistent. To test whether there is an association of the FTO locus with obesity and type 2 diabetes, we conducted a meta-analysis of 32 populations including 96,551 East and South Asians.
Methods
All studies published on the association between FTO-rs9939609 (or proxy [r 2 > 0.98]) and BMI, obesity or type 2 diabetes in East or South Asians were invited. Each study group analysed their data according to a standardised analysis plan. Association with type 2 diabetes was also adjusted for BMI. Random-effects meta-analyses were performed to pool all effect sizes.
Results
The FTO-rs9939609 minor allele increased risk of obesity by 1.25-fold/allele (p = 9.0 × 10−19), overweight by 1.13-fold/allele (p = 1.0 × 10−11) and type 2 diabetes by 1.15-fold/allele (p = 5.5 × 10−8). The association with type 2 diabetes was attenuated after adjustment for BMI (OR 1.10-fold/allele, p = 6.6 × 10−5). The FTO-rs9939609 minor allele increased BMI by 0.26 kg/m2 per allele (p = 2.8 × 10−17), WHR by 0.003/allele (p = 1.2 × 10−6), and body fat percentage by 0.31%/allele (p = 0.0005). Associations were similar using dominant models. While the minor allele is less common in East Asians (12–20%) than South Asians (30–33%), the effect of FTO variation on obesity-related traits and type 2 diabetes was similar in the two populations.
Conclusions/interpretation
FTO is associated with increased risk of obesity and type 2 diabetes, with effect sizes similar in East and South Asians and similar to those observed in Europeans. Furthermore, FTO is also associated with type 2 diabetes independently of BMI.
Similar content being viewed by others
Introduction
Large-scale genome-wide association studies (GWAS) in mainly white Europeans have identified at least 50 genetic loci to be robustly associated with obesity-related traits [1–12]. A cluster of common variants in the first intron of the fat mass and obesity-associated gene (FTO) was the first obesity-susceptibility locus to be identified by two independent GWAS in 2007 [1, 2] and has since been consistently replicated by many others and for a variety of obesity-related traits [7, 9, 13–15]. Of all currently identified obesity-susceptibility loci, the FTO locus has the most pronounced effect on BMI and obesity risk, at least in individuals of European descent. Each minor allele of any commonly investigated variant in FTO increases BMI by 0.30–0.40 kg/m2 (equivalent to 870–1,150 g for a person 1.7 m tall) and risk of obesity by ∼20% [7, 15]. The minor allele of the FTO variant is common (minor allele frequency (MAF)=∼42%) in white Europeans, such that 66% of Europeans carry at least one risk allele and 18% carry two risk alleles. Because of the high prevalence of the risk allele and its relatively strong effect on BMI, the FTO locus explains most (0.34%), yet little, of the variation in BMI in Europeans [7].
FTO has also been examined as an obesity-susceptibility locus in populations of non-white European origin. While the initial replication efforts in East Asian populations were inconsistent [16, 17], a growing number of studies have provided evidence that genetic variation in FTO influences BMI and obesity risk also in Chinese, Japanese, Korean and Filipino populations [18–27]. A GWAS for BMI in 7,861 Koreans identified variation in FTO (rs9939609) as the most significantly associated locus, nearly reaching genome-wide significance (p = 1.5 × 10−7) [28]. Furthermore, literature-based meta-analyses in Asians reported that the minor allele for the rs9939609 FTO single-nucleotide polymorphism (SNP) significantly (p = 9 × 10−9) increased the risk of obesity, but no other obesity-related traits were examined [18, 29, 30]. Fewer studies in South Asians have been reported, two of which confirmed the association between the FTO locus and obesity susceptibility [31, 32], whereas one did not [33]. The prevalence of the risk allele in East Asians (∼20%) and South Asians (∼30%) is substantially lower than in Europeans, and the reported effect sizes in both East and South Asians vary widely for BMI (OR 0.13–0.83 kg/m2 per minor allele) and obesity risk (OR 1.02–1.48 per minor allele) [16, 18, 20–25, 27, 34–39].
FTO was first identified as a type 2 diabetes-susceptibility gene, but, as further adjustment for BMI abolished the association with type 2 diabetes [1], it was suggested that FTO is primarily an obesity-susceptibility locus. However, the BMI-independent role of FTO in type 2 diabetes remains a matter of debate, particularly in Asians but also in white Europeans. While several studies have reported that the association between the FTO locus and risk of type 2 diabetes remained significant after adjustment for BMI [15, 18, 33, 35, 40, 41], others could not confirm this [21, 30, 32, 37, 42].
To firmly establish the association between the FTO locus and obesity susceptibility in East and South Asians and to assess its effect size and potential heterogeneity across Asian populations, we performed a systematic meta-analysis of data from 32 populations, including 96,551 men and women, using standardised study-specific association analyses. Furthermore, we examined whether the FTO locus is associated with type 2 diabetes independently of its association with BMI.
Methods
Literature search and study identification
We designed a meta-analysis based on de novo analyses of data according to a standardised plan to achieve the greatest consistency possible across studies. We identified all published studies (before September 2010) that had examined the association of genetic variation in FTO with risk of obesity and type 2 diabetes and with obesity-related continuous traits in East and South Asian adults (age ≥18 years) by a PubMed literature search using the key words ‘FTO’, ‘fat mass and obesity associated gene’ and ‘genome-wide association study’. References from the identified papers were subsequently screened to identify additional studies and to ensure that the list of eligible studies was complete. The literature search was carried out by two investigators independently, who cross-checked their search results for completeness.
Our literature search identified 38 publications, one of which was excluded because it was a subsample of another identified study. We invited the corresponding authors of the remaining 37 publications to join our meta-analysis, of which 26 agreed to participate and eventually 22 submitted raw data or summary statistics. We also included a Korean population with previously unpublished data (Y. M. Kim, J. Shin, C.B. Lee, M.K. Kim, Y. Tabara, T. Miki and B.Y. Choi), which was presented by a contributing author.
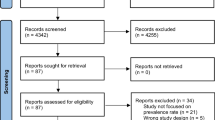
Taken together, our meta-analysis included data for 31 populations from 22 publications and one unpublished study, with 96,551 individuals altogether. The study identification and selection process is illustrated in Fig. 1.
All studies were conducted according to the Declaration of Helsinki. Informed consent was obtained from all participants, and the studies were approved by the ethics committees of the participating institutions.
Genotyping
The rs9939609 FTO SNP was examined in 18 studies, whereas proxy SNPs were used in 14 studies. More specifically, the rs8050135 SNP was genotyped in 11 studies of East Asians and one of South Asians, and the rs3751812 and rs17817449 SNP were each genotyped once in studies of East Asians (electronic supplementary material [ESM] Table 1). The linkage disequilibrium between rs9939609 and the three proxies (rs8050135, 3751812, rs17817449) is perfect (r 2 = 1) in populations of East Asian origin, based on CHB+JPT data from the HapMap (Rel 24/Phase II). The linkage disequilibrium between rs9939609 and rs8050135 in Indian Asians is very high (r 2 > 0.98), based on a subsample (n = 305) of the participating Lolipop study.
The genotyping success rate and concordance rate were >95%, and genotype distributions were in Hardy–Weinberg equilibrium (p > 0.01) in all participating studies (ESM Table 1).
Statistical analysis
As case–control definitions and statistical analyses used in the published papers were inconsistent, we asked analysts of each of the participating cohorts to re-analyse their data according to a standardised analysis plan. Summary statistics of each study were subsequently meta-analysed.
Obesity-susceptibility traits and type 2 diabetes
Overweight was defined as a BMI ≥24 kg/m2, and obesity as a BMI ≥28 kg/m2 according to the definition proposed by the Working Group on Obesity in China [43]. Anthropometric data, including weight, height, waist circumference, hip circumference and body fat percentage, were collected in each study as described previously (ESM Table 1), BMI was calculated as weight (kg) divided by height squared (m2), and WHR as waist circumference (cm) divided by hip circumference (cm). Raw data were used for analyses.
Type 2 diabetes was defined as meeting one or more of the following criteria: (1) fasting glucose ≥7.0 mmol/l; (2) 2-h glucose ≥11.1 mmol/l; (3) previous diagnosis of type 2 diabetes; (4) HbA1c ≥6.5% (48 mmol/mol); (5) self-reported type 2 diabetes (ESM Table 1).
Study-specific de novo data analyses
Association analyses within each study were performed for the total population and for men and women separately using additive and dominant genetic models. The associations of FTO-rs9939609 (or proxy) with risk of obesity and type 2 diabetes were assessed with multiple logistic regression models. Generalised linear models were used to assess the associations of FTO-rs9939609 (or proxy) with obesity-related continuous traits. In studies with a case–control design, analyses for continuous traits were conducted in control samples only. All analyses were adjusted for age and sex (sex-stratified analyses were only adjusted for age). The association with type 2 diabetes was also analysed with adjustment for BMI. Adjustments were performed by including the covariates (age, sex and/or BMI) as a linear term in the association model.
Summary statistics from the study-specific association analyses were reported in a standardised Excel form by the analysts of each study and collected centrally for meta-analyses.
Meta-analyses
Data extraction from the forms and meta-analyses was performed independently by two investigators and cross-checked for consistency. All ambiguities were clarified with the respective analysts before the final meta-analyses.
ORs and beta coefficients from the individual studies were pooled using DerSimonian and Laird random-effects meta-analyses [44]. Meta-analyses were performed of all studies combined. Because of differences in genetic background as well as in susceptibility to obesity and type 2 diabetes, meta-analyses were also stratified by East Asian and South Asian origin of the populations. Furthermore, East Asians were further stratified according to their country of origin.
Between-study heterogeneity was tested by Cochrane’s Q test and quantified by the I 2 index. I 2 values of <25%, 25–75% and >75% were defined as low, moderate and high heterogeneity, respectively [45]. To examine the sources of heterogeneity in our meta-analyses, we performed random-effects meta-regressions, where the between-study variance was estimated with the restricted maximum likelihood approach. Meta-regressions included the following study-specific variables as covariates: year of publication, country of origin, sample size, study design, mean age and mean BMI.
A funnel plot, along with Begg’s and Egger’s tests, was used to test for the presence of publication bias.
Statistical analyses were performed with the Stata 9.0 software (StataCorp LP, College Station, TX, USA). Meta-analyses and meta-regressions were implemented by the metan and metareg commands of Stata, respectively. p < 0.05 was considered to be significant, except for Cochrane’s Q test for heterogeneity and Begg’s and Egger’s tests for publication bias, where a level of p < 0.10 was used.
The variation in obesity-related continuous traits explained by the FTO variant was evaluated using the equation 2f(1−f)a 2, where f is the frequency of the variant and a is its additive standardised effect [5]. Population-attributable risk (PAR) was calculated as \( {\text{PAR}} = (X - {1})/X \). Assuming a multiplicative model, \( X = {\left( {{1} - f} \right)^{{2}}} + {2}f\left( {{1} - f} \right)\gamma + {f^{{2}}}{\gamma^2} \), where γ is the estimated OR, and f is the frequency of risk allele [46].
Results
Characteristics of populations included in the meta-analyses
Analyses were conducted in Chinese Hans (China Mainland: n = 10; Singapore: n = 2), Japanese (n = 7), Indians (n = 7), Koreans (n = 4), Singapore Malays (n = 1) and Filipinos (n = 1; Table 1). Fifteen of the populations were case–control designed for obesity (n = 3) or type 2 diabetes (n = 8) or both (n = 4), whereas 17 populations were population-based. The mean age and BMI of the populations ranged from 27.9 to 66.8 years and from 20.5 to 27.1 kg/m2, respectively. The prevalence in population-based studies ranged from 3.1% to 37.9% for obesity and from 2.9% to 41.9% for type 2 diabetes.
The MAF of FTO-rs9939609 (or proxy) is 12–14% in Chinese Hans and Koreans, 18–20% in Japanese and Filipinos, and 30–33% in Singapore Malays and Indians (Table 1).
Associations with obesity and overweight
A total of 24 populations (n obese = 13,032; n overweight = 22,474; n normalweight = 35,767) were available for meta-analyses of the association between the FTO variant and risk of obesity and overweight.
Each additional FTO-rs9939609 minor (A) allele increased the odds of obesity by 1.25 (p = 9.0 × 10−19) compared with normal weight individuals (Fig. 2), and by 1.17 (p = 7.4 × 10−11) compared with non-obese individuals (ESM Fig. 1). Each additional minor allele increased the odds of overweight by 1.13 (p = 1.0 × 10−11; ESM Fig. 2). The odds of obesity and overweight were the same in both East Asian and South Asian populations (p = 0.18 and 0.84, respectively; ESM Table 2). Associations were similar in men and women (ESM Table 3). The heterogeneity across all studies was low (13% ≤ I 2 ≤ 19%).
When a dominant genetic model was used, the odds were only slightly higher than for the additive genetic model (ESM Table 4).
Association with type 2 diabetes
In our meta-analysis of 22 populations (n cases = 33,744, n controls = 43,549), each additional FTO-rs9939609 minor allele increased the odds of type 2 diabetes by 1.15 (p = 5.5 × 10−8) when adjusted for age and sex (Fig. 3). Further adjustment for BMI attenuated, but did not abolish, the association with type 2 diabetes (OR 1.10, p = 6.6 × 10−5) (Fig. 4). Results were similar in East Asians and South Asians (ESM Table 2), in men and women (ESM Table 3), and when a dominant model was used (ESM Table 4).
The association results across all studies showed moderate heterogeneity (44% ≤ I 2 ≤ 48%; Figs 3 and 4). Meta-regression analyses revealed that the difference in study design contributed to some of the heterogeneity. Subsequent subgroup analyses showed that the association with type 2 diabetes was more pronounced in studies with a case–control design (OR [95% CI] = 1.19 [1.14, 1.23], p = 3.7 × 10−19, I 2 = 0.0%) than in cohort studies (OR [95% CI] = 1.09 [0.99, 1.20], p = 0.07, I 2 = 54.4%), which showed moderate heterogeneity (ESM Table 5).
Associations with obesity-related continuous traits
The meta-analyses of the association of FTO-rs9939609 with BMI, waist circumference, hip circumference, WHR and body fat percentage included 30 (n = 71,022), 22 (n = 51,543), 20 (n = 48,508), 20 (n = 48,508) and nine (n = 19,580) populations, respectively.
Each additional FTO-rs9939609 minor allele was associated with a 0.26 kg/m2 higher BMI (p = 2.8 × 10−17; equivalent to ∼750 g/allele for a person 1.7 m tall) (Fig. 5), 0.51 cm larger waist circumference (p = 3.0 × 10−9) (ESM Fig. 3), 0.36 cm larger hip circumference (p = 0.0003) (ESM Fig. 4), 0.003 greater WHR (p = 1.2 × 10−6; ESM Fig. 5), and 0.31% higher body fat percentage (p = 0.0005) (ESM Fig. 6). All associations were very similar between East and South Asians (ESM Table 2), between men and women (ESM Table 3), or when a dominant genetic model was used (ESM Table 4).
We observed moderate heterogeneity across studies in the associations with BMI and hip circumference (BMI: I 2 = 33%; hip circumference: I 2 = 51%; Fig. 5; ESM Fig. 4). Meta-regression suggested that, for BMI, the heterogeneity was mainly due to difference in mean age and mean BMI among different populations. For hip circumference, the heterogeneity was mainly attributed to difference in mean BMI, i.e. the effect of the FTO minor allele tended to be larger in populations with a mean BMI ≥24 kg/m2, compared with those with a mean BMI <24 kg/m2.
FTO-rs9939609 explained 0.16% and 0.20% of the inter-individual variation in BMI in East and South Asian populations, respectively. The proportion of variation in other obesity-related continuous traits explained by FTO-rs9939609 was <0.10% (ESM Table 2).
Publication bias
The funnel plots for the associations with obesity, type 2 diabetes, waist circumference, WHR and body fat percentage were symmetrical and the results for Begg’s and Egger’s tests were non-significant (p ≥ 0.10), indicating that our results were not affected by publication bias (ESM Fig. 7). However, there was some evidence of publication bias and/or genetic heterogeneity for BMI (Begg’s test, p = 0.08; Egger’s test, p = 0.07) and hip circumference (Begg’s test, p = 0.03; Egger’s test, p = 0.08; ESM Fig. 7).
Discussion
This meta-analysis, combining data of 96,551 Asians from 32 populations, further confirms that genetic variation in FTO is associated with increased risk of obesity in East and South Asians. Despite differences in genetic background and obesity susceptibility between East and South Asians, the effect of FTO on obesity and related traits was generally similar to, or only somewhat smaller than, those reported for white Europeans. We furthermore confirm that variation in FTO is associated with increased risk of type 2 diabetes, an association that, unlike in white Europeans, is not abolished after adjustment for BMI in both East and South Asians.
Large-scale studies in individuals of white European descent have reported that each additional FTO minor allele increases the odds of obesity by 1.20–1.32-fold [1, 5, 7, 47]. The association with obesity observed in Asians in the present study was remarkably similar, with each additional minor allele increasing obesity risk by 1.25-fold (95% CI 1.19, 1.31), consistent with the association observed for obesity in previous literature-based meta-analyses of case–control studies in East and South Asians [18, 29, 30].
The association of the FTO variant with overweight was the same in East and South Asians (OR 1.13 per minor allele) and very similar to the effects (ORs ranging from 1.13 to 1.18) that have been reported in large-scale studies of white Europeans [1, 7, 47]. While the effect sizes observed for the influence of FTO on obesity and overweight in Asians are very similar to those of Europeans, it should be noted that the definitions of obesity and overweight are different, as BMI cut-offs are somewhat lower in Asians than in Europeans, consistent with the association of BMI with metabolic disease [48].
The FTO minor allele increases BMI by 0.26 kg/m2 (equivalent to ∼750 g/allele for a person 1.7 m tall) in Asians, with very similar results for East and South Asians. This observation suggests that the effect of FTO on BMI in Asians is substantially smaller than the effect observed in a meta-analysis of more than 125,000 white Europeans (0.39 kg/m2 per minor allele, or 1,130 g per minor allele) [7]. This difference may be due to the fact that BMI in Asians does not represent exactly the same adiposity phenotype as in Europeans. However, given that other large-scale studies in white Europeans have reported effects for FTO on BMI that range between 0.26 and 0.39 kg/m2, the comparison between Asians and Europeans should be made with caution [1, 3, 5, 15, 47]. The FTO variant also showed convincing association with measures of fat distribution such as waist and hip circumference and WHR in Asians. Despite the often described difference in abdominal obesity between East and South Asians, the effect sizes were very similar in the two groups. Consistent with the observations for BMI, the effect sizes tended to be somewhat smaller than those reported for white Europeans. For example, each additional FTO minor allele increased waist circumference by 0.51 cm in Asians, whereas large-scale studies in Europeans have reported an increase of 0.73–1.00 cm [1, 9, 47].
As the MAF of the FTO variant is substantially lower in Asians (East Asians, ∼17%; South Asians, ∼32%) than in white Europeans (∼45%), and as the effect of this allele on obesity-related traits is similar or somewhat lower in Asians than in white Europeans, the overall contribution of genetic variation in FTO to obesity susceptibility will be lower in Asians, in particular East Asians. For example, the FTO variant explained less of the inter-individual variation in BMI in Asians (East Asians, 0.16%; South Asians, 0.20%) than in white Europeans (0.34%) [7]. Furthermore, the low risk allele frequency led to a lower PAR for the risk of obesity (East Asians, 8.3%; South Asians, 10.6%) and overweight (East Asians, 4.1%; South Asians, 7.8%) in Asians than in white Europeans (obesity, 20.4%; overweight, 12.7%) [1].
The FTO locus was first identified in a GWAS for type 2 diabetes in white Europeans, i.e. each minor allele increased the odds of diabetes by 1.15-fold [1]. However, after adjustment for BMI, the association between the FTO variant and type 2 diabetes was completely abolished (OR 1.03), suggesting that FTO is primarily an obesity-susceptibility locus [1]. In our meta-analysis, we observed a similar effect of FTO on risk of type 2 diabetes, with each minor allele increasing the odds by 1.15-fold. Interestingly, adjustment for BMI did not abolish the association, but only slightly attenuated it to a 1.10-fold increased risk of type 2 diabetes for each additional minor allele. These observations were similar in East and South Asians, suggesting that the FTO locus influences the risk of type 2 diabetes, at least in part, independently of its effect on BMI. The reason for the discrepancy between the original observations in Europeans and our observations in Asians are not known, but may be due to the fact that FTO seems to have a smaller effect on BMI in Asians than in Europeans. It may also be due to the fact that BMI, as suggested above, represents a different adiposity phenotype in Asians than in Europeans because of differences in body composition. Although BMI is a marker for general adiposity, it does not distinguish between fat mass and fat-free mass and does not reflect regional fat distribution. Observational studies have suggested that, for a given amount of total body fat, East and South Asians have more abdominal fat and less muscle mass than white Europeans [49, 50]. However, while it has been generally believed that in white Europeans the association with type 2 diabetes is fully mediated by the effect of FTO on BMI, not all studies confirm this observation. A recent large-scale study in 41,504 Scandinavians found that the FTO minor allele indeed increased type 2 diabetes risk (OR 1.13), but this association remained present (OR 1.09) after adjustment for BMI, consistent with the observations in the present study. The biological pathways that underlie the independent association between FTO variation with obesity and type 2 diabetes remain unclear. However, results of gene expression studies have shown that FTO expression in human islets cells is not associated with BMI [51], whereas FTO mRNA and protein levels in muscle are increased in individuals with type 2 diabetes compared with non-diabetic obese individuals or healthy lean controls [52]. Furthermore, FTO overproduction in myotubes suggested a role for FTO in oxidative metabolism, lipogenesis and oxidative stress in muscle, a cluster of metabolic defects characteristic of type 2 diabetes [52].
Despite the fact that our meta-analyses included Asians with different genetic backgrounds, the overall heterogeneity of the association effects was generally only low to moderate. Interestingly, we found that the associations were generally very similar in East and South Asians, although these populations are known to have genetically different origins [53]. Furthermore, we found no differences between men and women, consistent with the observations in white Europeans [7]. We found some evidence that age may contribute to the heterogeneity of the association between FTO and BMI. Life course effects have been reported in white Europeans [54], and longitudinal analyses will be needed to establish this in Asian populations. Longitudinal studies are also more appropriate than cross-sectional studies for disentangling the intricate interplay between FTO, obesity and type 2 diabetes throughout life [15].
It should be noted that the association between FTO variation and obesity risk in Asians had been established in three earlier meta-analyses [18, 29, 30]. These meta-analyses were substantially smaller than the present ones and focused solely on case–control analyses of obesity and type 2 diabetes, while no continuous traits were studied. The meta-analysis by Liu et al [18] included individuals of East and South Asian origin, which were analysed together without comparison of effect sizes between the two populations. This study also examined the association with type 2 diabetes, but did not explore the association after adjustment for BMI [18]. Furthermore, the three previous meta-analyses were all literature-based and thus more prone to publication bias, whereas our meta-analysis was designed on the basis of a de novo analysis of data according to a standardised plan in all studies identified as having available data and agreement to participate. No evidence of publication bias was observed except for the associations with BMI and hip circumference. The analytical consistency across studies helped minimise between-study heterogeneity. Although our results are representative of individuals of Southeast Asian, East Asian and South Asian descent, the association of FTO with risk of obesity and type 2 diabetes in other Asian populations remains to be examined.
In summary, we have firmly established that genetic variation in the first intron of FTO is associated with increased risk of obesity and type 2 diabetes in Asians, with effect sizes similar to those in Europeans. Furthermore, we confirm that the association of FTO with risk of type 2 diabetes is partly independent of BMI.
Abbreviations
- GWAS:
-
Genome-wide association study
- MAF:
-
Minor allele frequency
- PAR:
-
Population-attributable risk
- SNP:
-
Single-nucleotide polymorphism
References
Frayling TM, Timpson NJ, Weedon MN et al (2007) A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316:889–894
Scuteri A, Sanna S, Chen WM et al (2007) Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 3:e115
Loos RJ, Lindgren CM, Li S et al (2008) Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40:768–775
Willer CJ, Speliotes EK, Loos RJ et al (2009) Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41:25–34
Thorleifsson G, Walters GB, Gudbjartsson DF et al (2009) Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet 41:18–24
Chambers JC, Elliott P, Zabaneh D et al (2008) Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet 40:716–718
Speliotes EK, Willer CJ, Berndt SI et al (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42:937–948
Lindgren CM, Heid IM, Randall JC et al (2009) Genome-wide association scan meta-analysis identifies three loci influencing adiposity and fat distribution. PLoS Genet 5:e1000508
Heard-Costa NL, Zillikens MC, Monda KL et al (2009) NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS Genet 5:e1000539
Heid IM, Jackson AU, Randall JC et al (2010) Meta-analysis identifies 13 new loci associated with waist–hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42:949–960
Meyre D, Delplanque J, Chevre JC et al (2009) Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet 41:157–159
Scherag A, Dina C, Hinney A et al (2010) Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and German study groups. PLoS Genet 6:e1000916
Kring SI, Holst C, Zimmermann E et al (2008) FTO gene associated fatness in relation to body fat distribution and metabolic traits throughout a broad range of fatness. PLoS One 3:e2958
Hotta K, Nakamura M, Nakamura T et al (2010) Polymorphisms in NRXN3, TFAP2B, MSRA, LYPLAL1, FTO and MC4R and their effect on visceral fat area in the Japanese population. J Hum Genet 55:738–742
Hertel JK, Johansson S, Sonestedt E et al (2011) FTO, Type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes 60:1637–1644
Li H, Wu Y, Loos RJ et al (2008) Variants in the fat mass- and obesity-associated (FTO) gene are not associated with obesity in a Chinese Han population. Diabetes 57:264–268
Horikoshi M, Hara K, Ito C et al (2007) Variations in the HHEX gene are associated with increased risk of type 2 diabetes in the Japanese population. Diabetologia 50:2461–2466
Liu Y, Liu Z, Song Y et al (2010) Meta-analysis added power to identify variants in FTO associated with type 2 diabetes and obesity in the Asian population. Obesity (Silver Spring) 18:1619–1624
Karasawa S, Daimon M, Sasaki S et al (2010) Association of the common fat mass and obesity associated (FTO) gene polymorphism with obesity in a Japanese population. Endocr J 57:293–301
Hotta K, Nakata Y, Matsuo T et al (2008) Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet 53:546–553
Ng MC, Tam CH, So WY et al (2010) Implication of genetic variants near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with obesity and type 2 diabetes in 7705 Chinese. J Clin Endocrinol Metab 95:2418–2425
Tan JT, Dorajoo R, Seielstad M et al (2008) FTO variants are associated with obesity in the Chinese and Malay populations in Singapore. Diabetes 57:2851–2857
Chang YC, Liu PH, Lee WJ et al (2008) Common variation in the fat mass and obesity-associated (FTO) gene confers risk of obesity and modulates BMI in the Chinese population. Diabetes 57:2245–2252
Cheung CY, Tso AW, Cheung BM et al (2010) Obesity susceptibility genetic variants identified from recent genome-wide association studies: implications in a Chinese population. J Clin Endocrinol Metab 95:1395–1403
Cha SW, Choi SM, Kim KS et al (2008) Replication of genetic effects of FTO polymorphisms on BMI in a Korean population. Obesity (Silver Spring) 16:2187–2189
Lee HJ, Kim IK, Kang JH et al (2010) Effects of common FTO gene variants associated with BMI on dietary intake and physical activity in Koreans. Clin Chim Acta 411:1716–1722
Marvelle AF, Lange LA, Qin L, Adair LS, Mohlke KL (2008) Association of FTO with obesity-related traits in the Cebu Longitudinal Health and Nutrition Survey (CLHNS) Cohort. Diabetes 57:1987–1991
Cho YS, Go MJ, Kim YJ et al (2009) A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 41:527–534
Peng S, Zhu Y, Xu F, Ren X, Li X, Lai M (2011) FTO gene polymorphisms and obesity risk: a meta-analysis. BMC Med 9:71
Xi B, Mi J (2009) FTO polymorphisms are associated with obesity but not with diabetes in East Asian populations: a meta-analysis. Biomed Environ Sci 22:449–457
Dorajoo R, Blakemore AI, Sim X et al (2011) Replication of 13 obesity loci among Singaporean Chinese. Malay and Asian–Indian populations. Int J Obes (Lond). doi:10.1038/ijo.2011.86
Ramya K, Radha V, Ghosh S, Majumder PP, Mohan V (2010) Genetic variations in the FTO gene are associated with type 2 diabetes and obesity in south Indians (CURES-79). Diabetes Technol Ther 13:33–42
Yajnik CS, Janipalli CS, Bhaskar S et al (2009) FTO gene variants are strongly associated with type 2 diabetes in South Asian Indians. Diabetologia 52:247–252
Ng MC, Park KS, Oh B et al (2008) Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes 57:2226–2233
Li X, Song F, Jiang H et al (2010) A genetic variation in the fat mass- and obesity-associated gene is associated with obesity and newly diagnosed type 2 diabetes in a Chinese population. Diabetes Metab Res Rev 26:128–132
Shi J, Long J, Gao YT et al (2010) Evaluation of genetic susceptibility loci for obesity in Chinese women. Am J Epidemiol 172:244–254
Omori S, Tanaka Y, Takahashi A et al (2008) Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes 57:791–795
Tabara Y, Osawa H, Guo H et al (2009) Prognostic significance of FTO genotype in the development of obesity in Japanese: the J-SHIPP study. Int J Obes (Lond) 33:1243–1248
Han X, Luo Y, Ren Q et al (2010) Implication of genetic variants near SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, FTO, TCF2, KCNQ1, and WFS1 in type 2 diabetes in a Chinese population. BMC Med Genet 11:81
Sanghera DK, Ortega L, Han S et al (2008) Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet 9:59
Takeuchi F, Yamamoto K, Katsuya T et al (2011) Association of genetic variants for susceptibility to obesity with type 2 diabetes in Japanese individuals. Diabetologia 54:1350–1359
Shu XO, Long J, Cai Q et al (2010) Identification of new genetic risk variants for type 2 diabetes. PLoS Genet 6:e1001127
Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in China (2002) Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci 15:83–96
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Ng MC, Tam CH, Lam VK, So WY, Ma RC, Chan JC (2007) Replication and identification of novel variants at TCF7L2 associated with type 2 diabetes in Hong Kong Chinese. J Clin Endocrinol Metab 92:3733–3737
Li S, Zhao JH, Luan J et al (2009) Cumulative effects and predictive value of common obesity–susceptibility variants identified by genome-wide association studies. Am J Clin Nutr 91:184–190
Consultation WHOE (2004) Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363:157–163
Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL (2007) Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). Am J Clin Nutr 86:353–359
Chan JC, Malik V, Jia W et al (2009) Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301:2129–2140
Kirkpatrick CL, Marchetti P, Purrello F et al (2010) Type 2 diabetes susceptibility gene expression in normal or diabetic sorted human alpha and beta cells: correlations with age or BMI of islet donors. PLoS One 5:e11053
Bravard A, Lefai E, Meugnier E et al (2011) FTO is increased in muscle during type 2 diabetes, and its overexpression in myotubes alters insulin signaling, enhances lipogenesis and ROS production, and induces mitochondrial dysfunction. Diabetes 60:258–268
Consortium HP-AS, Abdulla MA, Ahmed I et al (2009) Mapping human genetic diversity in Asia. Science 326:1541–1545
Hardy R, Wills AK, Wong A et al (2009) Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet 19:545–552
Sha BY, Yang TL, Zhao LJ et al (2009) Genome-wide association study suggested copy number variation may be associated with body mass index in the Chinese population. J Hum Genet 54:199–202
Hu C, Zhang R, Wang C et al (2009) PPARG, KCNJ11, CDKAL1, CDKN2A-CDKN2B, IDE-KIF11-HHEX, IGF2BP2 and SLC30A8 are associated with type 2 diabetes in a Chinese population. PLoS One 4:e7643
Wen J, Ronn T, Olsson A et al (2010) Investigation of type 2 diabetes risk alleles support CDKN2A/B, CDKAL1, and TCF7L2 as susceptibility genes in a Han Chinese cohort. PLoS One 5:e9153
Cha S, Koo I, Park BL et al (2009) Genetic effects of FTO and MC4R polymorphisms on body mass in constitutional types. Evid Based Complement Alternat Med. doi:10.1093/ecam/nep162
Takeuchi F, Serizawa M, Yamamoto K et al (2009) Confirmation of multiple risk loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes 58:1690–1699
Acknowledgements
A full list of acknowledgements can be found in the ESM. This work was supported by the following: Hong Kong Government Research Grants Council Central Allocation Scheme (CUHK 1/04C), Research Grants Council Earmarked Research Grant (CUHK4724/07M) and the Innovation and Technology Fund of the Government of the Hong Kong SAR (ITS/487/09FP); National Institutes of Health (DK078150, TW05596, HL085144, TW008288) and pilot funds (RR20649, ES10126, and DK56350); National Institutes of Health (R01 AR050496, R21 AG027110, R01 AG026564, R01 AR057049-01A1, R21 AA015973) and Specialized Center of Research (P50 AR055081) and National Science Foundation of China (81000363, 31000554) and the PhD Programs Foundation of Ministry of Education of China (20100201120058) and the Fundamental Research Funds for the Central Universities, Shanghai Leading Academic Discipline Project (S30501); National Research Foundation of Korea (NRF, no. 20100020617); the Japanese Society for the Promotion of Science (17209021) and of Priority Area ‘Applied Genomics’ (1601223, 17019028, 18018020, 20018026); the Korea Health 21 R&D Project, Korean Ministry of Health & Welfare (00-PJ3-PG6-GN07-001); the National Institute for Health Research Comprehensive Biomedical Research Centre at Imperial College Healthcare NHS Trust, the British Heart Foundation (SP/04/002), the Medical Research Council (G0700931), the Wellcome Trust (084723/Z/08/Z) and the National Institute for Health Research (RP-PG-0407-10371); the Knowledge Innovation Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (2009KIP401), the Chief Scientist Program of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (SIBS2008006), the Knowledge Innovation Program Project of the Chinese Academy of Sciences (KSCX1-YW-02), the National Natural Science Foundation of China (30930081), and National High Technology Research and Development Program (863 Program) (2009AA022704), National 973 Program (2011CB504002); the National Science Council (NSC 95-3112-B002-002, NSC 96-2752-B002-008-PAE) of Taiwan; major program of Shanghai Municipality for Basic Research (08dj1400601), National Natural Science Foundation of China (30800617); National Institute of Health (NIH/FIC KO1 TW006087, NIH/NIDDK R01 DK082766); the Program for the National Natural Science Foundation of China (NSFC-30872116) and New Century Excellent Talents in the University of China (NCET-04-0707); Council of Scientific and Industrial Research, Government of India (NWP0032); the National Heart, Lung, and Blood Institute, National Institutes of Health, US Department of Health and Human Services (N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, 44221) and the National Institute of Diabetes and Digestive and Kidney Diseases R01 (DK062290) from the National Institutes of Health.
Contribution statement
RJFL, HL and XL contributed to the conception and design of the study. CL and TOK performed the literature search, designed the analysis plan, performed the meta-analyses and researched the data. HL, TOK, CL and RJFL wrote the manuscript. HL, JZ, YL, CH, ZY, WZ, WB, SC, YW, TY, AS, BYC, CSY, DZ, FT, KY, JCC, KRM, LFB, MI, EN, NL, TF, SK, WW, CVJ, WL, YC, YX, YG, SL, YS, SHK, HDS, KSP, CHDF, JYK, PCS, KSLL, WZ, XS, HD, HI, GVK, DKS, LC, LL, RH, YK, MD, KH, WJ, JSK, JCC, GRC, RCM, SM, RD, MY, RT, NK, XL and RJFL collected study-specific data, analysed the study-specific data according to the standardised analysis plan, and reviewed and edited the manuscript. All authors have approved the final version of the manuscript to be published.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Acknowledgements
(PDF 69 kb)
ESM Figure 1
(PDF 113 kb)
ESM Figure 2
(PDF 113 kb)
ESM Figure 3
(PDF 109 kb)
ESM Figure 4
(PDF 111 kb)
ESM Figure 5
(PDF 103 kb)
ESM Figure 6
(PDF 94 kb)
ESM Figure 7
(PDF 49 kb)
ESM Table 1
Information on definition of type 2 diabetes and genotyping quality control (PDF 103 kb)
ESM Table 2
Associations of FTO rs9939609 (or proxy SNP) with obesity, overweight, type 2 diabetes and obesity-related continuous traits in East Asians and South Asians separately, assuming an additive genetic model (PDF 33 kb)
ESM Table 3
Associations of FTO rs9939609 (or proxy SNP) with obesity, overweight, type 2 diabetes and obesity-related continuous traits in men and women separately, assuming an additive genetic model (PDF 15 kb)
ESM Table 4
Associations of FTO rs9939609 (proxy SNPs) with obesity, overweight, type 2 diabetes and obesity-related continuous traits, assuming a dominant genetic model (PDF 29 kb)
ESM Table 5
Subgroup analyses for the associations of FTO rs9939609 (proxy SNPs) with type 2 diabetes, BMI and hip circumference (PDF 76 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Li, H., Kilpeläinen, T.O., Liu, C. et al. Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 55, 981–995 (2012). https://doi.org/10.1007/s00125-011-2370-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2370-7