Abstract
While canopy temperature (CT) shows a strong and reliable association with yield under drought and heat stress and is used in wheat breeding to select for yield, little is known of its genetic control. The objective of this study was to determine the gene action controlling CT in five wheat populations grown in diverse environments (heat, drought, and well-irrigated conditions). CT showed negative phenotypic correlations with grain yield under drought and well-irrigated environments. Additive × additive effects were most prevalent and significant for all crosses and environments. Dominance and dominance × dominance gene actions were also found, though the significance and direction was specific for each environment and genotypic cross. The use of CT as a selection criterion to improve tolerance to drought was supported by its significant association with grain yield and the genotype differences observed between cultivars. Our results indicated that genetic gains for CT in wheat could be achieved through conventional breeding. However, given some dominance and epistatic effects, it would be necessary to delay the selection process until the frequency of heterozygous loci within families is reduced.
Similar content being viewed by others
Introduction
Wheat (Triticum aestivum L) is commonly grown in regions where yields are limited by low seasonal rainfall. Under water-limited conditions, the efficiency in the selection of drought-tolerant genotypes based on empirical selection for yields per se is restricted by the low heritability of yield as well as by a large genotype by environment interaction (Trethowan et al. 2002). In addition, yield evaluation in early generations is difficult because yield per plant may not be related to crop yield. Progress through plant breeding has been achieved by using physiological traits in the selection process to complement conventional breeding for yield (Araus 1996, 2003; Condon et al. 2002; Richards 1996; Richards et al. 2002; Reynolds et al. 2009).
The physiological trait preferred for breeding for high-yield following an indirect approach must reveal genetic variability in different populations, as well as high genetic correlations with yield and heritability large enough to permit genetic gains based on selection (Araus et al. 2002; Richards et al. 2002). Screening techniques for those traits must be rapid, accurate, easy to operate, preferably non-destructive, repeatable, and with reasonable costs (Araus 1996), to complement the breeder eye. New screening tools are necessary to be tested in terms of their ability to express the genetic variation in the population, with minimum environmental influence and low genotype by environment interactions (Condon and Richards 1992).
Canopy temperature (CT) has been used as a screening tool for predicting high wheat yield in rainfed environments (Araus et al. 2002, 2003; Blum et al. 1989; Condon and Richards 1992; Olivares-Villegas et al. 2007). Since leaf temperature is depressed below air temperature when water evaporates, CT is an indirect measure of the (instantaneous) transpiration at the whole-crop level (Reynolds et al. 2001) and plant water status (Araus et al. 2003). Drought-susceptible genotypes showed warmer canopies than water-stress tolerant cultivars (Reynolds et al. 2001). The potential of CT for screening wheat genotypes under moisture-stress conditions (Rashid et al. 1999) is based on its significant correlations with grain yield (Reynolds et al. 2001) and the genotype differences observed between cultivars (Amani et al. 1996; Araus et al. 2003; Reynolds et al. 1994). The analysis is cheap, fast, non-destructive, and easy to use for monitoring of whole-plant response to water stress. However, CT reliability was questioned when evaluated in different environments (Royo et al. 2002; Sojka et al. 1981).
It is expected that new drought-tolerant varieties will both increase and stabilize yields from year to year. Grain yield is largely determined by the amount of water the crop extracts from the soil and the efficiency of water use during biomass accumulation and grain development. A cultivar able to use more water during its growth would have lower CT, mainly as a result of more open stomata. Genotypic variation has been reported for CT in wheat (Amani et al. 1996; Ayeneh et al. 2002; Blum et al. 1989; Fischer et al. 1998; Rashid et al. 1999; Reynolds et al. 1994), although little is known about gene action for CT in bread wheat.
The objective of this study was to determine the gene action for CT in five bread wheat populations grown under diverse environments (heat, drought, and well-irrigated conditions). Progenies derived from crosses between high and low CT genotypes were used in a generation mean-based mating design. Understanding gene action for CT would increase the efficiency and rate of progress through plant breeding for water-limited environments by helping in the selection of strategies for efficient breeding.
Materials and methods
Genotypes
Genotypes came from CIMMYT’s spring bread wheat (Triticum aestivum L) breeding program for dry areas (Trethowan and Reynolds 2007). The wheat populations used for this study were obtained by crossing SW89.5193/Attila (Cross 1), Weebill4//Gim/Lira (Cross 2), Weebill4/SW89.5193 (Cross 3), SW89.5193/Prinia (Cross 4) and Weebill4/Prinia (Cross 5). Parents (P1 and P2) for each cross were chosen according to their contrasting CT. Weebill4 is a Babax-derived spring bread wheat generated by CIMMYT which combines high-yield performance and good adaptation. This cultivar was selected as the low CT parent. Both Prinia and Attila showed intermediate values for CT. High CT parents, SW89.5193 and Gim/Lira, are both well-adapted high-yielding spring bread cultivars.
Crosses were made during 2003–2004 at Ciudad Obregon, Mexico. F1s along with parents were grown under screenhouse conditions in El Batan, Mexico, and self pollinated to produce F2 seed. The F1 plants were also back-crossed to P1 and P2 to produce back-crossed populations BC1P1 and BC1P2, respectively. F2 seeds were harvested from F1 plants, and planted as individual plants in screenhouse conditions in El Batan, Mexico (Cross 1, 2, and 3) and in field conditions in Ciudad Obregon, Mexico (Cross 4 and 5). Seeds from each F2 plant were bulked and planted in small plots, resulting in 142, 82, 105, 139, and 155 F2 derived groups for crosses 1–5, respectively. Each F2 derived group was self pollinated, as indicated in Table 1.
Table 1 shows a description of cross pedigrees, cycles, locations, generations, and activities performed for the five populations created to evaluate gene action for canopy temperature.
Growth conditions
Plants of the parental lines, F1, backcrosses, and F2 derived groups were grown at the CIMMYT Experimental Station near Ciudad Obregon, NW Mexico (27 20° N, 109 54° W, 38 m asl) during three wheat cycles (2005–2006, 2006, and 2006–2007) under three different environments (well-irrigated, drought, and heat stress) (Table 1). The site is a temperate, high radiation, irrigated environment. Meteorological data are summarized in Table 2.
Well-irrigated and drought experiments were grown during the spring wheat season (late November sowing and April harvest) and delayed sowing (late February sowing and June harvest) was used for the heat stressed experiments. Seed were planted as plots of 2 m long and 0.75 m wide consisting of one raised bed with 2 rows/bed and 15 cm between rows (Limón-Ortega et al. 2000). Average sowing density was 83 kg ha−1. Planting rates of 10–15 g of seed per plot were used to achieve at least 250 plants per plot.
The experiment followed an α-lattice design (Falconer 1989) with two replicates. Fertilization was applied to avoid yield limitations. Weed, disease, and pest control were applied as necessary. Plants in well-irrigated plots used an estimated 600 mm of water during the complete growing cycle. Under drought, approximate total moisture available was 200 mm. Available soil water was gravimetrically estimated using samples from adjacent plots. For the well-irrigated treatment, plots were irrigated when approximately 50% of available soil moisture was depleted according to gravimetric determinations. The well-irrigated plots received a total of five irrigations during both 2005–2006 and 2006–2007. Drought treatment consisted of four irrigation events in 2005–2006 and three irrigation events in the 2006–2007 cycle. For the heat stress environment, a total of five irrigations were applied during the 2006 cycle.
Agronomic and physiological measurements
Canopy temperature measurements were taken during vegetative/boot stage using a hand-held infrared thermometer (Mikron Infrared, USA). The thermometer averaged the temperature readings observed while running the instrument along the plot at a moderate speed (3–5 s per plot). Field measurements were taken not earlier than 11 am and not later than 3 pm (Reynolds et al. 1994), to avoid dew and to evaluate the plants when air water demand is maximum. Windy and cloudy days were avoided (Reynolds et al. 2001). For each population, CT was evaluated several times (1–6) during the vegetative stage. The number of times CT was registered was limited by the irrigation schedule, pesticides applications, and inappropriate weather conditions. When physiological maturity was reached, plots were harvested by combines and their grain yields weighed.
Statistical analysis
Analysis of variance was conducted using the mixed linear model procedure MIXED (SAS 2004). Genotypes, environments (well-irrigated, drought, and heat stress), years, and the interaction between main factors were considered as fixed effects while replicates, incomplete blocks within replicates, and incomplete blocks within replicates and environments were considered as random effects. Tests of significance of fixed effects were accomplished by using appropriate mean squares (Steel et al. 1997).
Quantitative genetic analysis of phenotypic data for five populations consisting of six generations (P1, P2, F1, F2-derived, BC1P1 and BC1P2) was performed using SASQuant computer program in SAS (Gusmini et al. 2007). Generation means and variances were combined to estimate the gene effects (Mather and Jinks 1971; Foolad and Lin 2001). Additive, dominance, and the various epistatic effects were partitioned according to Hayman’s generation mean analysis procedure (Gamble 1962; Hayman 1958). Main genetic components consisted of additive variance [a] and dominance variance [d]. Interaction components (epistasis) were described as additive × additive [aa], additive × dominance [ad], and dominance × dominance [dd].
The estimated parameters were used to test the goodness of fit of different genetic models (Rowe and Alexander 1980). We tested nine genetic models, with the general mean [m], [a], [d], and a maximum of two interaction components to retain at least one degree of freedom. We accepted the models that fitted the data (chi-square > 0.05). Thus, models with interaction components (epistatic effects) were considered if the chi-square-test was significant at P < 0.05.
Significance of each individual genetic component was tested using Student’s t test. Genetic components with t test <0.05 were considered different from zero and significant to the model.
Results
Relationship between CT and grain yield
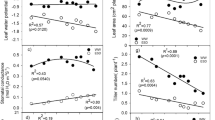
There was a negative correlation between CT and grain yield (well-irrigated 2005–2006: r = −0.16, P = 0.015; drought 2005–2006: r = −0.34, P < 0.0001; 2006–2007: r = −0.75, P < 0.001, Fig. 1). The relationship found between CT and grain yield was proved to be repeatable across diverse environments and years (Fig. 1) which agree with the results of previous studies on the robustness of the association between this physiological character and grain yield (Balota et al. 2007; Blum et al. 1989; Olivares-Villegas et al. 2007).
Results from ANOVA
Significant phenotypic variation for CT was observed in the populations derived from crosses between parents of contrasting CT values grown in diverse environments. The analyses of variance performed for each population over environments and years are indicated in Table 3. The magnitude of the main effects and interactions varied between the five crosses. Genotype by environment interactions were significant for three out of five populations (Table 3). Mean values for CT of parents, F1, BC1P1, BC1P2 and F2 derived groups for each environment and for the two seasons 2005–2006 and 2006–2007 are presented in Table 4.
The mean values of F1s were similar to calculated mid-parent values in most of the cases, although both positive and negative deviations for some F1s were observed. Those deviations suggested heterosis and the presence of some non-additive/epistatic gene action for CT. As expected, in most of the situations the backcrosses increased the frequency of alleles from the recurrent parent resulting in a change of the CT values in the direction of that recurrent parent.
Weebill4, the cultivar selected as the low CT parent, had the lowest CT when averaged over all environments and years. SW89.5193 on the contrary, had the highest CT, which corresponded with the criteria used for its selection as high CT-high-yielding parent. These differences in CT of the parents resulted in differences among progeny generations.
Considering Cross 1 (SW89.5193/Attila), there was a significant effect for genotype, year, and genotype by environment interaction. Comparing the parent CTs, SW89.5193 had equal or higher CT than Attila in all three environments. CTs of the F1s were close to mid-parent values for the heat environment and both the well-irrigated and drought 2006–2007, while deviations towards the high CT parent were observed under drought 2005–2006. The evidence of F1 mid-parent deviation under drought suggested some dominance or epistatic gene action under this environment. Backcross CTs tended to approach the mean of the recurrent parent, except in drought 2005–2006.
The main effect of genotype and year, as well as all the interactions including year, were significant for CTs from Cross 2 (Weebill4//Gim/Lira). Weebill4 showed equal CT than Gim/Lira. CTs for F1 were close to mid-parent values. CTs for BC1P1, and BC1P2 tended to approach the low CT parent.
In Cross 3 (Weebill4/SW89.5193), genotype and year effects, as well as genotype by environment and genotype by year interaction were significant. Significant differences between generations were observed in the CTs in this cross. Deviations from the mid-parent value were observed for F1 CTs under both drought and well-irrigated environments. The direction of this deviation was different across years and environments. Backcross CT values tended to approach the mean of the recurrent parent.
In Cross 4 (SW89.5193/Prinia), genotype and environment main effects and genotype by year interaction were significant. Opposite deviations from the mid-parent value were observed for F1s under well-irrigated and drought conditions in 2005–2006. Under well-irrigated environments CT for the F1 tended to be lower than the mid-parent value, while it was higher under drought. Deviations were also observed for the backcross CTs, which did not correspond with the mean of the recurrent parent in this specific cross.
In Cross 5 (Weebill4/Prinia), genotype main effect was not significant. The genotype by environment interaction was significant in this cross as generation groups changed their relative performance under well-irrigated and drought conditions. F1 CTs were close to mid-parent value under drought and close to highest parent under well-irrigated conditions. Under drought, backcross CTs tended to be closer to the performance of the recurrent parents. However, both BC1P1 and BC1P2 did not differ in the well-irrigated environment and were closer to the high CT parent.
Goodness of fit of genetic models
The goodness of fit of the genetic components to a total of nine genetic models for CT were tested for each cross, year and environment. The selected nine models represent all possible combinations having the general mean [m], [a], [d], and a maximum of two interaction components to retain at least one degree of freedom. The goodness of fit chi-square values and probabilities are showed in Table 5. The chi-square value tested the null hypothesis that the data fit the model, thus chi-square values with P ≥ 0.05 denoted goodness of fit.
Under drought, the model having the [m], [a], [d], [aa] and [ad] genetic components was selected because it had the lowest chi-square and highest P values in four out of five crosses in 2005–2006 and in one out of three crosses in 2006–2007. The model explained by [m], [a], [d], [aa] and [dd] was selected for the other crosses grown under drought.
Considering the well-irrigated environment, the model explained by [m], [a], [d], [aa] and [dd] was selected in three out of five crosses in 2005–2006 and in three out of three crosses in 2006–2007. On other hand, the model described by [m], [a], [d], [aa] and [ad] was selected for the other crosses grown under well-irrigated conditions.
For the cross evaluated under heat, the model containing the [m], [a], [d], [aa] and [dd] parameters showed the best goodness of fit.
Generalizing across environments and crosses, there was a repetitive pattern in the models with the lowest chi-square values, namely m[a][d][aa][ad] and m[a][d][aa][dd], suggesting that models containing the interaction components [aa] and either [ad] or [dd] resulted in the best fit of the data.
Significance of individual genetic components
Significance of each individual genetic component was tested using Student’s t test (Table 6), since not all the components in the selected models could be significant, even when the models were selected as having the lowest chi-square. Genetic components with t test <0.05 were considered different from zero and significant to the model.
The mean values for CT were highly significant in all the situations tested. The [aa] epistatic component was significant in 10 out of the 17 combination of crosses, environments and years analyzed. The [dd] component was significant in 4 out of those 17 combinations, while the [d] component was significant in 2 combinations. Thus, excluding the [m] value, estimates of [aa] were consistently having the highest significance levels.
Discussion
The models described as m[a][d][aa][ad] and m[a][d][aa][dd] consistently showed the best goodness of fit of the data. However, not all the components in the selected models were significant. Excluding the [m] values, estimates of [aa] consistently had the highest significance levels. Thus, considering both the genetic model goodness of fit and the significance of each of the genetic parameters in the model, [aa] genetic effect was identified as the single most important component of the genetic variance for CT. Additive × additive epistatic effects indicate the need to sample potentially larger populations (Lande and Thompson 1990) in order to recover desired combinations, even though, alleles could be pyramided for decreasing CT in a target plant ideotype. It has been proposed that delaying selection until lines are homozygous should reduce the need for large population sizes (Rebetzke et al. 2006).
The complexity in CT heredity has been suggested to be associated with the fact that different cultivars would have different mechanisms to adapt to particular environmental conditions (Liu et al. 2005). The absence of a significant [a] effect could then be the result of a complex gene pathway for CT, involving several genes of small effect with different expression under diverse environments (Mathews et al. 2008). Interactions among genes, stress adaptive traits, and environment would contribute to reduce this additive component. While digenic epistatic quantitative trait loci (QTLs) were recognized to affect CT in rice, with a total of 16 pairs of significant interactions associated with this trait (Liu et al. 2005), efforts are now being concentrated in the identification of QTL for CT in wheat (Pinto et al. 2008).
We have chosen to work with F2 derived material to be able to assess both dominance and additive effects. But this has also impacted on the precision of the estimation of the various genetic components. Because loci are heterozygous at F2, phenotypic variation is quite compressed compared to what it is at a more fixed level, in the absence of strong dominance effects. Populations derived from F4 or F5 plants would have contained more homozygous lines at a given loci or at combinations of loci and therefore would have allowed a more precise estimation of additive and additive × additive effects. Therefore, results could have been different with more homozygous populations.
The negative sign of the [aa] values and the significance of models explained by [a], [d] and [aa] epistasis has also been demonstrated by Rebetzke et al. (2003) for the expression of leaf conductance measured prior to anthesis on irrigated plants from different generations of three wheat crosses. Additionally, a negative sign on the additive gene effect was also reported for carbon isotope discrimination (CID), a variable correlated with transpiration efficiency (Rebetzke et al. 2006). They concluded that additive alleles for reduced CID could have been mainly transmitted for single and interacting loci from the low CID parent.
The similarities observed between the level and significance of epistatic effects found when comparing the results of the CT study with those on both leaf conductance and CID would suggest that these traits are under oligogenic or polygenic control, thus, the substitution for desired alleles would depend on the average effects of specific alleles at interacting loci. The gene action patterns reported for traits related with water uptake and transpiration efficiency suggested that additive alleles may be largely transmitted by single and interacting loci from the parents expressing high water-use efficiency. That would support the crossing strategy proposed by Reynolds et al. (2009) where parents with potentially complementary traits are selected to ensure continued genetic gains in breeding for drought and heat stressed environments.
Both additive and non-additive gene action were important in controlling expression of CT in the different crosses and environments evaluated. As explained by Milus and Line (1986), the lack of fit observed in some of the models may be explained by more complex genetic control, linkage of interacting loci, or a large environmental variance. For example, dominance and dominance × dominance effects were significant in some of the situations tested but not in a repetitive pattern. Presence of dominance genetic effects would indicate that selection for CT should be delayed until after inbreeding is obtained and the frequency of heterozygous loci within families has decreased. However, dominance effects could be exploited in the development of F1 hybrids.
Reynolds et al. (2007) reported theoretical yield gains associated with CT under diverse environments and demonstrated by principal component analysis and regression analysis that traits associated with water use were making the strongest contribution to yield. Canopy temperature has been identified as the single most drought-adaptive trait contributing to a higher performance in a Seri–Babax RIL wheat population grown under different water regimes and environments (Olivares-Villegas et al. 2007). In this population, low canopy temperatures and higher grain yields were associated with specific linkage groups by QTL analyses (Pinto et al. 2008). Further genomic and transcriptomic studies would be necessary to permit the identification of parents to introduce in the breeding program and the detection of proper allelic combinations by marker-assisted selection. However, complex traits—such as CT—could result from the interaction of diverse genetic pathways which would make difficult the identification of QTLs, especially under diverse environments and stresses (Mathews et al. 2008).
Conclusions
Our results confirmed the suitability of CT as a selection criterion to improve tolerance to stress, based on the associations between CT and grain yield in addition to the genotype differences observed between cultivars. Additive × additive and dominance × dominance interactions were greater than their corresponding additive and dominance components in all crosses and environments indicating that epistasis was a significant contributor to genetic variances. The presence of epistatic gene action indicates that the expression of a gene at one locus could be masked by a gene at another locus, though the finding of significant additive effects indicates that improvement can be made by conventional breeding. The partitioning of total epistasis revealed that additive × additive gene effects were highly significant for CT. The dominance and epistatic gene action effects for CT suggest that the selection may be delayed until having low heterozygous loci within families in order to increase the efficiency in the selection methods for yield improvement through CT.
References
Amani I, Fischer RA, Reynolds MP (1996) Canopy temperature depression association with yield of irrigated spring wheat cultivars in a hot climate. J Agron Crop Sci 176:199–229
Araus JL (1996) Integrative physiological criteria associated with yield potential. In: Reynolds MP, Rajaram S, McNab A (eds) Increasing yield potential in wheat: Breaking the barriers. CIMMYT, Mexico, pp 150–166
Araus JL (2003) Breeding cereals for Mediterranean conditions: ecophysiological clues for biotechnology applications. Ann Appl Biol 142:129–141
Araus JL, Slafer GA, Reynolds MP, Royo C (2002) Plant breeding and drought in C-3 cereals: what should we breed for? Ann Bot 89:925–940
Araus JL, Villegas D, Aparicio N, del Moral LFG, El Hani S, Rharrabti Y, Ferrio JP, Royo C (2003) Environmental factors determining carbon isotope discrimination and yield in durum wheat under Mediterranean conditions. Crop Sci 43:170–180
Ayeneh A, van Ginkel M, Reynolds MP, Ammar K (2002) Comparison of leaf, spike, peduncle, and canopy temperature depression in wheat under heat stress. Field Crops Res 79:173–184
Balota M, Payne WA, Evett SR, Lazar MD (2007) Canopy temperature depression sampling to assess grain yield and genotypic differentiation in winter wheat. Crop Sci 47:1518–1529
Blum A, Shpiler L, Gozlan G, Mayer J (1989) Yield stability and canopy temperature of wheat genotypes under drought stress. Field Crops Res 22:289–296
Condon AG, Richards RA (1992) Broad-sense heritability and genotype × environment interaction for carbon isotope discrimination in field-grown wheat. Aust J Agric Res 43:921–934
Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2002) Improving intrinsic water-use efficiency and crop yield. Crop Sci 42:122–131
Falconer DS (1989) Introduction to quantitative genetics. Wiley, New York
Fischer RA, Rees D, Sayre KD, Lu Z-M, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38:1467–1475
Foolad MR, Lin GY (2001) Genetic analysis of cold tolerance during vegetative growth in tomato, Lycopersicon esculentum Mill. Euphytica 122:105–111
Gamble EE (1962) Gene effects in corn (Zea mays L) I. Separation and relative importance of gene effects for yield. Can J Plant Sci 42:339–348
Gusmini G, Wehner TC, Donaghy SB (2007) SASQuant: a SAS software program to estimate genetic effects and heritabilities of quantitative traits in populations consisting of 6 related generations. J Hered 98:345–350
Hayman BI (1958) The separation of epistatic from additive and dominance variation in generation means. Heredity 12:371–390
Lande R, Thompson R (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124:743–756
Limón-Ortega A, Sayre KD, Francis CA (2000) Wheat and maize yields in response to straw management and nitrogen under a bed-planting system. Agron J 92:295–302
Liu H, Zou G, Liu G, Hu S, Li M, Yu X, Mei H, Luo L (2005) Correlation analysis and QTL identification for canopy temperature, leaf water potential and spikelet fertility in rice under contrasting moisture regimes. Chin Sci Bull 50:317–326
Mather K, Jinks JL (1971) Biometrical genetics. Chapman and Hall, London
Mathews KL, Malosetti M, Chapman S, McIntyre L, Reynolds M, Shorter R, van Eeuwijk F (2008) Multi-environment QTL mixed models for drought stress adaptation in wheat. Theor Appl Genet 117:1077–1091
Milus EA, Line RF (1986) Gene action for inheritance of durable, high-temperature, adult-plant resistance to stripe rust in wheat. Phytopathology 76:435–441
Olivares-Villegas JJ, Reynolds MP, McDonald GK (2007) Drought-adaptive attributes in the Seri/Babax hexaploid wheat population. Funct Plant Biol 34:189–203
Pinto S, Chapman SC, McIntyre CL, Shorter R, Reynolds M (2008) QTL for canopy temperature response related to yield in both heat and drought environments. In: Appels R, Eastwood R, Lagudah E, Langridge P, Mackay M, McIntyre L, Sharp P (eds) Proceedings of the 11th international wheat genetics symposium. Brisbane, Australia
Rashid A, Stark JC, Tanveer A, Mustafa T (1999) Use of canopy temperature measurements as a screening tool for drought tolerance in spring wheat. J Agron Crop Sci 182:231–237
Rebetzke GJ, Condon AG, Richards RA, Farquhar GD (2003) Genetic control of leaf conductance in three wheat crosses. Aust J Agric Res 54:381–387
Rebetzke GJ, Richards RA, Condon AG, Farquhar GD (2006) Inheritance of reduced carbon isotope discrimination in bread wheat (Triticum aestivum L.). Euphytica 150:97–106
Reynolds MP, Balota M, Delgado MIB, Amani I, Fischer RA (1994) Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Aust J Plant Physiol 21:717–730
Reynolds MP, Ortiz-Monasterio JI, McNab A (2001) Application of physiology in wheat breeding. CIMMYT, Mexico, pp 124–135
Reynolds MP, Saint Pierre C, Saad ASI, Vargas M, Condon AG (2007) Evaluating potential genetic gains in wheat associated with stress-adaptive trait expression in elite genetic resources under drought and heat stress. Crop Sci 47:S-172–S-189
Reynolds M, Manes Y, Izanloo A, Langridge P (2009) Phenotyping approaches for physiological breeding and gene discovery in wheat. Ann Appl Biol 155:309–320
Richards RA (1996) Defining selection criteria to improve yield under drought. Plant Growth Regul 20:157–166
Richards RA, Rebetzke GJ, Condon AG, van Herwaarden AF (2002) Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci 42:111–121
Rowe KE, Alexander WL (1980) Computations for estimating the genetic parameters in joint-scaling tests. Crop Sci 20:109–110
Royo C, Villegas D, García del Moral LF, Elhani S, Aparicio N, Rharrabti Y, Araus JL (2002) Comparative performance of carbon isotope discrimination and canopy temperature depression as predictors of genotype differences in durum wheat yield in Spain. Aust J Agric Res 53:561–569
SAS Institute (2004) SAS Institute, Cary
Sojka RE, Stolzy HL, Fischer RA (1981) Seasonal drought response of selected wheat cultivars. Agron J 73:838–845
Steel RGD, Torrie JH, Dickey DA (1997) Principles and procedures of statistics, a biometrical approach. McGraw-Hill, New York
Trethowan RM, Reynolds MP (2007) Drought resistance: genetic approaches for improving productivity under stress. In: Buck HT, Nisi JE, Salomón N (eds) Wheat production in stressed environments. Springer, The Netherlands, pp 289–299
Trethowan RM, van Ginkel M, Rajaram S (2002) Progress in breeding wheat for yield and adaptation in global drought affected environments. Crop Sci 42:1441–1446
Acknowledgments
The authors thank Rubeena Shaikh for her contribution in the development of the wheat populations used in this study; Greg Rebetzke for his assistance in the experimental design; Gregorio Alvarado for his statistical assistance, and Araceli Torres Garcia, Jose Luis Barrios Gonzalez, and Eugenio Perez Dorame for technical greenhouse/field support. This work was financially supported by the Grains Research and Development Corporation (GRDC), Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Langridge.
Rights and permissions
About this article
Cite this article
Saint Pierre, C., Crossa, J., Manes, Y. et al. Gene action of canopy temperature in bread wheat under diverse environments. Theor Appl Genet 120, 1107–1117 (2010). https://doi.org/10.1007/s00122-009-1238-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-1238-4