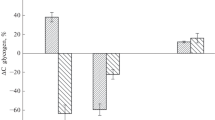
Abstract
Glycogen is a vital energy substrate for anaerobic organisms, and the size of glycogen stores can be a limiting factor for anoxia tolerance of animals. To this end, glycogen stores in 12 different tissues of the crucian carp (Carassius carassius L.), an anoxia-tolerant fish species, were examined. Glycogen content of different tissues was 2–10 times higher in winter (0.68–18.20% of tissue wet weight) than in summer (0.12–4.23%). In scale, bone and brain glycogen stores were strongly dependent on body mass (range between 0.6 and 785 g), small fish having significantly more glycogen than large fish (p < 0.05). In fin and skin, size dependence was evident in winter, but not in summer, while in other tissues (ventricle, atrium, intestine, liver, muscle, and spleen), no size dependence was found. The liver was much bigger in small than large fish (p < 0.001), and there was a prominent enlargement of the liver in winter irrespective of fish size. As a consequence, the whole body glycogen reserves, measured as a sum of glycogen from different tissues, varied from 6.1% of the body mass in the 1-g fish to 2.0% in the 800-g fish. Since anaerobic metabolic rate scales down with body size, the whole body glycogen reserves could provide energy for approximately 79 and 88 days of anoxia in small and large fish, respectively. There was, however, a drastic difference in tissue distribution of glycogen between large and small fish: in the small fish, the liver was the major glycogen store (68% of the stores), while in the large fish, the white myotomal muscle was the principal deposit of glycogen (57%). Since muscle glycogen is considered to be unavailable for blood glucose regulation, its usefulness in anoxia tolerance of the large crucian carp might be limited, although not excluded. Therefore, mobilization of muscle glycogen under anoxia needs to be rigorously tested.





Similar content being viewed by others
References
Almeida-Val VMF, Val AL, Duncan WP, Souza FCA, Paula-Silva MN, Land S (2000) Scaling effects on hypoxia tolerance in the Amazon fish Astronotus ocellatus (Perciformes: Cichlidae): contribution of tissue enzyme levels. Comp Biochem Physiol B 125:219–226
Björntorp P, Sjöström L (1978) Carbohydrate storage in man: speculations and some quantitative considerations. Metabolism 12(suppl 2):1853–1865
Chou JY, Matern D, Mansfield BC, Chen YT (2002) Type I glycogen storage diseases: disorders of the glucose-6-phosphatase complex. Curr Mol Med 2:121–143
Clarke A, Johnston NM (1999) Scaling metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Everett MV, Crawford DL (2010) Adaptation versus allometry: population and body mass effects on hypoxic metabolism in Fundulus grandis. Physiol Biochem Zool 83:182–190
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Goolish EM, Adelman IR (1988) Tissue-specific allometry of an aerobic respiratory enzyme in a large and a small species of cyprinid (Teleostei). Can J Zool 66:2199–2208
Greenberg CC, Jurczak MJ, Danos AM, Brady MJ (2006) Glycogen branches out: new perspectives on the role of glycogen metabolism in the integration of metabolic pathways. Am J Physiol 291:E1–E8
Holopainen IJ, Hyvärinen H (1985) Ecology and physiology of crucian carp (Carassius carassius (L.)) in small Finnish ponds with anoxic conditions in winter. Verh Internat Verein Limnol 22:2566–2570
Holopainen IJ, Hyvärinen H, Piironen J (1986) Anaerobic wintering of crucian carp (Carassius carassius L.)—II. Metabolic products. Comp Biochem Physiol A 83:239–242
Huang MT, Lee CF, Lin RF, Chen RJ (1997) The exchange between proglycogen and macroglycogen and the metabolic role of the protein-rich glycogen in rat skeletal muscle. J Clin Invest 99:501–505
Huidekoper HH, Visser G, Ackermans MT, Sauerwein HP, Wijburg FA (2010) A potential role for muscle in glucose homeostasis: in vivo kinetic studies in glycogen storage disease type 1a and fructose-1, 6-bisphosphatase deficiency. J Inherit Metab Dis 33:25–31
Hyvärinen H, Holopainen IJ, Piironen J (1985) Anaerobic wintering of crucian carp (Carassius carassius L.)—I. Annual dynamics of glycogen reserves in nature. Comp Biochem Physiol A 82:797–803
Johnston IA, Bernard LM (1983) Utilization of the ethanol pathway in carp following exposure to anoxia. J Exp Biol 104:73–78
Lo S, Russell JC, Taylor AW (1970) Determination of glycogen in small tissue samples. J Appl Physiol 28:234–236
Matikainen N, Vornanen M (1992) Effect of season and temperature acclimation on the function of crucian carp (Carassius carassius) heart. J Exp Biol 167:203–220
Nilsson GE (1992) Evidence for a role of GABA in metabolic depression during anoxia in crucian carp (Carassius carassius). J Exp Biol 164:243–259
Nilsson GE (2001) Surviving anoxia with the brain turned on. NIPS 16:217–221
Nilsson GE, Östlund-Nilsson S (2008) Does size matter for hypoxia tolerance in fish? Biol Rev 83:173–189
Piironen J, Holopainen IJ (1986) A note on seasonality in anoxia tolerance of crucian carp (Carassius carassius L.) in the laboratory. Ann Zool Fenn 23:335–338
Previs SF, Brunengraber DZ, Brunengraber H (2009) Is there glucose production outside of the liver and kidney? Annu Rev Nutr 29:43–57
Roach JP (2002) Glycogen and its metabolism. Curr Mol Med 2:101–120
Schöndorff B, Wachholder K (1914) Über den Glykogenstoffwechsel der Fische. I. Der Glykogengehalt von Süsswasserfischen. Pflugers Arch 157:147–164
Shieh JJ, Pan CJ, Mansfield BC, Chou JY (2004) A potential new role for muscle in blood glucose homeostasis. J Biol Chem 279:26215–26219
Sollid J, De Angelis P, Gundersen K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J Exp Biol 206:3667–3673
Tattersall GJ, Ultsch GR (2008) Physiological ecology of aquatic overwintering in ranid frogs. Biol Rev 83:119–140
Tiitu V, Vornanen M (2001) Cold adaptation suppresses the contractility of both atrial and ventricular muscle of the crucian carp (Carassius carassius L.) heart. J Fish Biol 59:141–156
Valtonen T (1974) Seasonal and sex-bound variation in the carbohydrate metabolism of the liver of the whitefish. Comp Biochem Physiol A 47:713–727
Van den Thillart G, Kesbeke F, Waarde A (1980) Anaerobic energy-metabolism of goldfish, Carassius auratus (L.). J Comp Physiol B 136:45–52
Van Waversveld J, Addink ADF, van den Thillart G, Smit H (1988) Direct calorimetry on free swimming goldfish at different oxygen levels. J Therm Anal 33:1019–1026
Van Waversveld J, Addink A, van den Thillart G (1989) Simultaneous direct and indirect calorimetry on normoxic and anoxic goldfish. J Exp Biol 142:325–335
Vornanen M (1994) Seasonal adaptation of crucian carp (Carassius carassius L.) heart: glycogen stores and lactate dehydrogenase activity. Can J Zool 72:433–442
Vornanen M, Paajanen V (2006) Seasonal changes in glycogen content and Na+-K+-ATPase activity in the brain of crucian carp. Am J Physiol 291:R1482–R1489
Vornanen M, Stecyk J, Nilsson GE (2009) The anoxia-tolerant crucian carp (Carassius carassius L.). In: Richards JG, Farrell AP, Brauner CJ (eds) Hypoxia, vol. 27. Elsevier, Amsterdam, pp 397–441
Wachholder K (1923) Uber den Glykogengehalt der Fische. II. Mitteilung. Der Einfluss der Jahreszeit auf den Glykogengehalt. Pflugers Arch 199:528–530
Walker RM, Johansen PH (1977) Anaerobic metabolism in goldfish (Carassius auratus). Can J Zool 55:1304–1311
Winberg GG (1960) Rate of metabolism and food requirements of fishes. Fish Res Board Can Transl Ser 194:1–202
Acknowledgements
This study was supported by a grant (No. 14795) from the Academy of Finland to MV. Anita Kervinen is appreciated for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vornanen, M., Asikainen, J. & Haverinen, J. Body mass dependence of glycogen stores in the anoxia-tolerant crucian carp (Carassius carassius L.). Naturwissenschaften 98, 225–232 (2011). https://doi.org/10.1007/s00114-011-0764-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-011-0764-5