Abstract
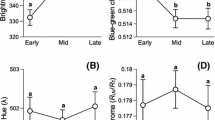
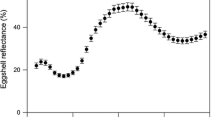
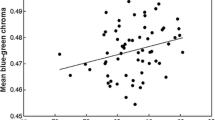
How colouration provides information about individuals in birds has been a central issue in recent decades. Although much information has been derived, little is known about the adaptive significance of egg colouration in birds. A recent idea suggests that biliverdin- and porphyrin-pigmented eggs may act as a post-mating sexual signal for males to assess female quality. In birds, it is common for males to influence prelaying female condition by courtship feeding. Using Eurasian kestrels, a species that lays protoporphyrin-pigmented eggs, we descriptively assessed the influence of male feeding on egg pigmentation by considering female phenotype, condition, breeding parameters and male body condition. We found that older females and females with greyer tails (an index of individual quality) produce highly pigmented eggs. However, male body condition was the only variable that explained egg colouration when considered together with the female-related variables. Therefore, females that mated with males in better condition laid highly pigmented eggs. With the same species, we also explored the cost of producing protoporphyrin-pigmented eggs using a food-supply experiment before the laying period. Food supplementation did not increase egg pigmentation, but hatching success and egg mass were positively related to egg colouration only in food supplied pairs. We suggest that egg colouration might be costly to produce and probably suggests egg quality. However, this cost cannot be explained by female quality, but by male condition instead. In general, our results do not support the theory that egg colouration is a post-mating sexual signal in species where males determine female condition at the time of laying.


Similar content being viewed by others
References
Aitken R (1940) Routes of transhumance on the Spanish Meseta: review. Geogr J 106:59–69
Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G (2004) Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett 7:363–368
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Aparicio JM (1994) The seasonal decline in clutch size: an experiment with supplementary food in the Kestrel, Falco tinnunculus. Oikos 71:451–458
Aparicio JM (1999) Intraclutch egg-size variation in the Eurasian kestrel: advantages and disadvantages of hatching from large eggs. Auk 116:825–830
Bitton PP, O'Brien EL, Dawson RD (2007) Plumage brightness and age predict extrapair fertilization success of male tree swallows, Tachycineta bicolor. Anim Behav 74:1777–1784
Blanco G, Martínez-Padilla J, Dávila JA, Serrano D, Viñuela J (2003) First evidence of sex differences in the duration of avian embryonic period: consequences for sibling competition in sexually dimorphic birds. Behav Ecol 14:702–706
Cassey P, Ewen JG, Blackburn TM, Hauber ME, Vorobyev M, Justin Marshall N (2008) Eggshell colour does not predict measures of maternal investment in eggs of Turdus thrushes. Naturwissenschaften 95:713–721
Darwin CR (1871) Descent of man, and selection in relation to sex. John Murray, London
Deeming DC (2002) Avian incubation. Behaviour, environment, and evolution. Oxford University Press, New York
Doutrelant C, Gregoire A, Grnac N, Gomez D, Lambrechts MM, Perret P (2008) Female coloration indicates female reproductive capacity in blue tits. J Evol Biol 21:226–233
Faivre B, Gregoire A, Preault M, Cezilly F, Sorci G (2003) Immune activation rapidly mirrored in a secondary sexual trait. Science 300:103
Fargallo JA, Blanco G, Potti J, Viñuela J (2001) Nestbox provisioning in a rural population of Eurasian Kestrels: breeding performance, nest predation and parasitism. Bird Study 48:236–244
Fargallo JA, Laaksonen T, Korpimäki E, Wakamatsu K (2007a) A melanin-based trait reflects environmental growth conditions of nestling male Eurasian kestrels. Evol Ecol 21:157–171
Fargallo JA, Martínez-Padilla J, Toledano-Díaz A, Santiago-Moreno J, Dávila JA (2007b) Sex and testosterone effects on growth, immunity and melanin coloration of nestling Eurasian kestrels. J Anim Ecol 76:201–209
Gosler AG, Higham JP, Reynolds SJ (2005) Why are birds' eggs speckled? Ecol Lett 8:1105–1113
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds: a role for parasites? Science 218:384–387
Hargitai R, Herényi M, Török J (2008) Eggshell coloration in relation to male ornamentation, female condition and egg quality in the collared flycatcher Ficedula albicollis. J Avian Biol 39:413–422
Helfenstein F, Wagner RH, Danchin E, Rossi J (2003) Functions of courtship feeding in black-legged kittiwakes: natural and sexual selection. Anim Behav 65:1027–1033
Hill GE, McGraw KJ (2006) Bird coloration. Function and evolution. Harvard University Press, London
Jagannath A, Shore RF, Walker LA, Ferns PN, Gosler AG (2008) Eggshell pigmentation indicates pesticide contamination. J Appl Ecol 45:133–140
Kendeigh SC (1952) Parental care and its evolution in birds. Illiniois Biological Monographs 22:1–357
Krebs JR (1970) The efficiency of courtship feeding in the blue tit Parus caeruleus. Ibis 112:108–112
Krist M, Grim T (2007) Are blue eggs a sexually selected signal of female collared flycatchers? A cross-fostering experiment. Behav Ecol Sociobiol 61:863–876
Kurtz J, Kalbe M, Langefors A, Mayer I, Milinski M, Hasselquist D (2007) An experimental test of the immunocompetence handicap hypothesis in a teleost fish: 11-ketotestosterone suppresses innate immunity in three-spined sticklebacks. Am Nat 170:509–519
Lack D (1940) Courtship feeding in birds. Auk 57:169–178
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lifjeld JT, Slagsvold T (1986) The function of courtship feeding during incubation in the pied flycatcher Ficedula hypoleuca. Anim Behav 34:1441–1453
López-Rull I, Celis P, Gil D (2007) Egg colour covaries with female expression of a male ornament in the spotless starling (Sturnus unicolor). Ethology 113:926–933
Loyau A, Gómez D, Moureu BT, Thery M, Hart NS, Saint Jalme M, Bennett ATD, Sorci G (2007) Iridescent structurally based coloration of eyespots correlates with mating success in the peacock. Behav Ecol 18:1123–1131
Lundberg A, Alatalo RV (1992) The Pied Flycatcher. T&AD Poyser
Markert JA, Arnegard ME (2007) Size-dependent use of territorial space by a rock-dwelling cichlid fish. Oecologia 154:611–621
Martínez-Padilla J (2006) Prelaying maternal condition modifies the association between egg mass and T-cell-mediated immunity in kestrels. Behav Ecol Sociobiol 60:510–515
Martínez-Padilla J, Fargallo JA (2007) Food supply during prelaying period modifies the sex-dependent investment in eggs of Eurasian kestrels. Behav Ecol Sociobiol 61:1735–1742
Martínez-Padilla J, Mougeot F, Pérez-Rodrıíguez L, Bortolotti GR (2007) Nematode parasites reduce carotenoid-based signalling in male red grouse. Biol Lett 3:161–164
Meijer T, Masman D, Daan S (1989) Energetics of reproduction in female kestrels. Auk 106:549–559
Morales J, Sanz JJ, Moreno J (2006) Egg colour reflects the amount of yolk maternal antibodies and fledging success in a songbird. Biol Lett 2:334–336
Moreno J, Osorno JL (2003) Avian egg color and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806
Moreno J, Osorno JL, Morales J, Merino S, Tomás G (2004) Egg colouration and male parental effort in the pied flycatcher Ficedula hypoleuca. J Avian Biol 35:300–304
Moreno J, Morales J, Lobato E, Merino S, Tomás G, de la Puente J (2006) More colourful eggs induce a higher relative paternal investment in the pied flycatcher Ficedula hypoleuca: a cross-fostering experiment. J Avian Biol 37:555–560
Mougeot F, Thibault JC, Bretagnolle V (2002) Effects of territorial intrusions, courtship feedings and mate fidelity on the copulation behaviour of the osprey. Anim Behav 64:759–769
Mougeot F, Piertney SB, Leckie F, Evans S, Moss R, Redpath SM, Hudson PJ (2005a) Experimentally increased aggressiveness reduces population kin structure and subsequent recruitment in red grouse Lagopus lagopus scoticus. J Anim Ecol 74:488–497
Mougeot F, Redpath SM, Piertney S, Hudson PJ (2005b) Separating behavioral and physiological mechanisms in testosterone-mediated trade-offs. Am Nat 166:158–168
Mougeot F, Pérez-Rodríguez L, Martínez-Padilla J, Leckie FM, Redpath SM (2007) Parasites, testosterone and honest carotenoid-based signaling of health. Funct Ecol 21:886–898
Newton I (1979) Population ecology of raptors. T & AD Poyser, London
Nisbet ICT (1973) Courtship feeding, egg size and breeding success in common terns. Nature 241:141–142
Olsson M, Madsen T, Wapstra E, Silverin B, Ujvari B, Wittzell H (2005) MHC, health, color, and reproductive success in sand lizards. Behav Ecol Sociobiol 58:289–294
Palokangas P, Korpimäki E, Hakkarainen H, Huhta E, Tolonen P, Alatalo RV (1994) Female kestrels gain reproductive success by choosing brightly ornamented males. Anim Behav 47:443–448
Punzalan D, Rodd FH, Rowe L (2008) Sexual selection mediated by the thermoregulatory effects of male colour pattern in the ambush bug Phymata americana. P Roy Soc B-Biol Sci 275:483–492
Reynolds SJ, Martin GB, Cassey P (2009) Is sexual selection blurring the functional significance of eggshell coloration hypotheses? Anim Behav 78:209–215
Royama T (1966) A re-interpretation of courtship feeding. Bird Study 13:116–129
Salzer DW, Larkin GJ (1990) Impact of courtship feeding on clutch and third-egg size in glaucous-winged gulls. Anim Behav 39:1149–1162
Sanz JJ, García-Navas V (2009) Eggshell pigmentation pattern in relation to breeding performance of blue tits Cyanistes caeruleus. J Anim Ecol 78:31–41
Soler JJ, Møller AP (2007) A comparative analysis of the evolution of variation in appearance of eggs of European passerines in relation to brood parasitism. Behav Ecol 7:89–94
Soler JJ, Navarro C, Pérez Contreras T, Avilés JM, Cuervo JJ (2008) Sexually selected egg coloration in Spotless starlings. Am Nat 171:183–194
Solis JC, de Lope F (1995) Nest and egg crypsis in the ground-nesting stone curlew Burhinus oedicnemus. J Avian Biol 26:135–138
Steele RH (1986) Courtship feeding in Drosophila subobscura. I. The nutritional significance of courtship feeding. Anim Behav 34:1087–1098
Stevens M, Parraga CA, Cuthill IC, Partridge JC, Troscianko TS (2007) Using digital photography to study animal colouration. Biol J Linn Soc 90:211–237
Stokke BG, Moksnes A, Roskaft E (2002) Obligate brood parasites as selective agents for evolution of egg appearance in passerine birds. Evolution 56:199–205
Tasker CR, Mills JA (1981) A functional analysis of courtship feeding in the red-billed gull, Larus novaehollandiae scopulinus. Behaviour 77:221–241
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Cambridge
Underwood TJ, Sealy SG (2002) Adaptive significance of egg coloration. In: Deeming DC (ed) Avian incubation, behaviour, environment and evolution. University Press, Oxford, pp. 280–298
Vahed K (1998) The function of nuptial feeding in insects-review of empirical studies. Biol Rev 73:43–78
Velando A, Torres R, Espinosa I (2005) Male coloration and chick condition in bluefooted booby: a cross-fostering experiment. Behav Ecol Sociobiol 58:175–180
Vergara P, de Neve L, Fargallo JA (2007) Agonistic behaviour prior to laying predicts clutch size in Eurasian kestrels: an experiment with natural decoys. Anim Behav 74:1515–1523
Vergara P, Fargallo JA, Banda E, Parejo D, Lemus JA, García-Montijano M (2008) Low frequency of anti-acetylcholinesterase pesticidepoisoning in lesser and Eurasian kestrels of Spanish grassland and farmland populations. Biol Conserv 141:499–505
Vergara P, Fargallo JA, Martínez-Padilla J, Lemus JA (2009) Inter-annual variation and information content of melanin-based coloration in female Eurasian kestrels. Biol J Linn Soc 97:781–790
Village A (1990) The kestrel. T & AD Poyser, London
Viñuela J (2000) Opposing selective pressures on hatching asynchrony: egg viability, brood reduction, and nestling growth. Behav Ecol Sociobiol 48:333–343
Zahavi A (1975) Mate selection—a selection for a handicap. J Theor Biol 53:205–214
Acknowledgements
JM-P wishes to dedicate this paper to his friend Aurelio Pérez, who recently passed away, to his passion, for encouraging him and for what he undoubtedly did for wildlife conservation. We thank the Finat family for kindly allowing us to conduct the study on their property and C. Marqués and J. San Teodoro for their collaboration in the field. The Association for the Study of Animal Behaviour (ASAB), J. Viñuela and G. Blanco partially funded the field work. Sarah Young and Jessica Haines greatly improved the English. L.P.R. was supported by a postdoctoral contract from the Junta de Comunidades de Castilla-La Mancha.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Padilla, J., Dixon, H., Vergara, P. et al. Does egg colouration reflect male condition in birds?. Naturwissenschaften 97, 469–477 (2010). https://doi.org/10.1007/s00114-010-0660-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-010-0660-4