Abstract
Type 1 diabetes (T1D) is an autoimmune disease characterised by the destruction of the insulin-producing beta (β)-cells within the pancreatic islets. We have previously identified a novel parasite-derived molecule, termed Fasciola hepatica helminth defence molecule 1 (FhHDM-1), that prevents T1D development in non-obese diabetic (NOD) mice. In this study, proteomic analyses of pancreas tissue from NOD mice suggested that FhHDM-1 activated the PI3K/Akt signalling pathway, which is associated with β-cell metabolism, survival and proliferation. Consistent with this finding, FhHDM-1 preserved β-cell mass in NOD mice. Examination of the biodistribution of FhHDM-1 after intraperitoneal administration in NOD mice revealed that the parasite peptide localised to the pancreas, suggesting that it exerted a direct effect on the survival/function of β-cells. This was confirmed in vitro, as the interaction of FhHDM-1 with the NOD-derived β-cell line, NIT-1, resulted in increased levels of phosphorylated Akt, increased NADH and NADPH and reduced activity of the NAD-dependent DNA nick sensor, poly(ADP-ribose) polymerase (PARP-1). As a consequence, β-cell survival was enhanced and apoptosis was prevented in the presence of the pro-inflammatory cytokines that destroy β-cells during T1D pathogenesis. Similarly, FhHDM-1 protected primary human islets from cytokine-induced apoptosis. Importantly, while FhHDM-1 promoted β-cell survival, it did not induce proliferation. Collectively, these data indicate that FhHDM-1 has significant therapeutic applications to promote β-cell survival, which is required for T1D and T2D prevention and islet transplantation.
Key messages
-
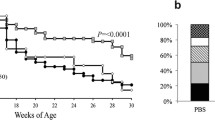
FhHDM-1 preserves β-cell mass in NOD mice and prevents the development of T1D.
-
FhHDM-1 enhances phosphorylation of Akt in mouse β-cell lines.
-
FhHDM-1 increases levels of NADH/NADPH in mouse β-cell lines in vitro.
-
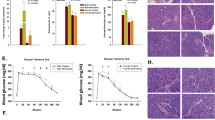
FhHDM-1 prevents cytokine-induced cell death of mouse β-cell lines and primary human β-cells in vitro via activation of the PI3K/Akt pathway.






Similar content being viewed by others
Availability of data and material
This manuscript has data included as electronic supplementary material.
Abbreviations
- PI3K/Akt:
-
Phosphoinositide 3-kinase/protein kinase B
- SGK1:
-
Serum/glucocorticoid-regulated kinase 1
- THBS1:
-
Thrombospondin 1
- IGF1R:
-
Insulin-like growth factor 1 receptor
- FBP1:
-
Fructose bisphosphatase 1
- AKR1B7:
-
Aldo-keto reductase family 1, member B7
- NOD:
-
Non-obese diabetic
- VC:
-
Vehicle control
- PARP:
-
Poly-ADP-ribose polymerase
- IP:
-
Intraperitoneal
- PBS:
-
Phosphate-buffered saline
- LC–MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- FBS:
-
Foetal bovine serum
- GO:
-
Gene ontology
- CM:
-
Pro-inflammatory cytokine mix
- HRP:
-
Horseradish peroxidase
- ECL:
-
Enhanced chemiluminescence
- TUNEL:
-
Terminal deoxynucleotidyl transferase dUTP nick end labelling
- PI:
-
Propidium iodide
- GLP:
-
Glucagon-like peptide-1
- GIP:
-
Glucose-dependent insulinotropic polypeptide
- FOXO:
-
Forkhead box O
- CRAMP:
-
Cathelicidin-related antimicrobial peptide
- EGFR:
-
Epidermal growth factor receptor
- ROI:
-
Reactive oxygen intermediates
- NADH:
-
Nicotinamide adenine dinucleotide reduced form
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate reduced form
References
Roep BO, Thomaidou S, van Tienhoven R, Zaldumbide A (2021) Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat Rev Endocrinol 17:150–161. https://doi.org/10.1038/s41574-020-00443-4
Daneman D (2006) Type 1 diabetes. The Lancet 367:847–858. https://doi.org/10.1016/S0140-6736(06)68341-4
Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT (2000) Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes 49:1–7. https://doi.org/10.2337/diabetes.49.1.1
Turley S, Poirot L, Hattori M, Benoist C, Mathis D (2003) Physiological β cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 198:1527–1537. https://doi.org/10.1084/jem.20030966
Roep BO, Peakman M (2010) Surrogate end points in the design of immunotherapy trials: emerging lessons from type 1 diabetes. Nat Rev Immunol 10:145–152. https://doi.org/10.1038/nri2705
Eizirik DL, Colli ML, Ortis F (2009) The role of inflammation in insulitis and [beta]-cell loss in type 1 diabetes. Nat Rev Endocrinol 5:219–226. https://doi.org/10.1038/nrendo.2009.21
Jun H-S, Yoon C-S, Zbytnuik L, Van Rooijen N, Yoon J-W (1999) The role of macrophages in T cell–mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med 189:347–358. https://doi.org/10.1084/jem.189.2.347
Kay T, Chaplin H, Parker J, Stephens L, Thomas H (1997) CD4+ and CD8+ T lymphocytes: clarification of their pathogenic roles in diabetes in the NOD mouse. Res Immunol 148:320–327. https://doi.org/10.1016/s0923-2494(97)87241-0
Thomas HE, Kay TW (2000) Beta cell destruction in the development of autoimmune diabetes in the non-obese diabetic (NOD) mouse. Diabetes Metab Res Rev 16:251–261. https://doi.org/10.1002/1520-7560(200007/08)16:4%3c251::aid-dmrr126%3e3.0.co;2-c
Zhao Y, Scott NA, Quah HS, Krishnamurthy B, Bond F, Loudovaris T, Mannering SI, Kay TWH, Thomas HE (2015) Mouse pancreatic beta cells express MHC class II and stimulate CD4+ T cells to proliferate. Eur J Immunol 45:2494–2503. https://doi.org/10.1002/eji.201445378
Kroger CJ, Clark M, Ke Q, Tisch RM (2018) Therapies to suppress β cell autoimmunity in type 1 diabetes. Front Immunol 9:1891. https://doi.org/10.3389/fimmu.2018.01891
Coppieters KT, Harrison LC, von Herrath MG (2013) Trials in type 1 diabetes: Antigen-specific therapies. Clin Immunol (Orlando, Fla.) 149:345–355. https://doi.org/10.1016/j.clim.2013.02.002
Ludvigsson J, Krisky D, Casas R, Battelino T, Castaño L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert J-J et al (2012) GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med 366:433–442. https://doi.org/10.1056/NEJMoa1107096
Kaufman A, Herold KC (2009) Anti-CD3 mAbs for treatment of type 1 diabetes. Diabetes Metab Res Rev 25:302–306. https://doi.org/10.1002/dmrr.933
Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS et al (2019) An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 381:603–613. https://doi.org/10.1056/NEJMoa1902226
Sims EK, Bundy BN, Stier K, Serti E, Lim N, Long SA, Geyer SM, Moran A, Greenbaum CJ, Evans-Molina C et al (2021) Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med 13. https://doi.org/10.1126/scitranslmed.abc8980
Robinson MW, Alvarado R, To J, Hutchinson AT, Dowdell SN, Lund M, Turnbull L, Whitchurch CB, O’Brien BA, Dalton JP (2012) A helminth cathelicidin-like protein suppresses antigen processing and presentation in macrophages via inhibition of lysosomal vATPase. FASEB J 26:4614–4627. https://doi.org/10.1096/fj.12-213876
Robinson MW, Donnelly S, Hutchinson AT, To J, Taylor NL, Norton RS, Perugini MA, Dalton JP (2011) A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLoS Pathog 7:e1002042. https://doi.org/10.1371/journal.ppat.1002042
Lund ME, Greer J, Dixit A, Alvarado R, McCauley-Winter P, To J, Tanaka A, Hutchinson AT, Robinson MW, Simpson AM et al (2016) A parasite-derived 68-mer peptide ameliorates autoimmune disease in murine models of type 1 diabetes and multiple sclerosis. Sci Rep 6:37789. https://doi.org/10.1038/srep37789
Lund ME, O’Brien BA, Hutchinson AT, Robinson MW, Simpson AM, Dalton JP, Donnelly S (2014) Secreted proteins from the helminth Fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PLoS One 9:e86289. https://doi.org/10.1371/journal.pone.0086289
Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74:5383–5392. https://doi.org/10.1021/ac025747h
Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75:4646–4658. https://doi.org/10.1021/ac0341261
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Kanehisa M, Goto S (2000) KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res 28:27–30. https://doi.org/10.1093/nar/28.1.27
Putt KS, Beilman GJ, Hergenrother PJ (2005) Direct quantitation of poly(ADP-ribose) polymerase (PARP) activity as a means to distinguish necrotic and apoptotic death in cell and tissue samples. ChemBioChem 6:53–55. https://doi.org/10.1002/cbic.200400330
Tuttle RL, Gill NS, Pugh W, Lee J-P, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ (2001) Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat Med 7:1133–1137. https://doi.org/10.1038/nm1001-1133
Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA (2001) Islet β cell expression of constitutively active Akt1/PKBα induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Investig 108:1631. https://doi.org/10.1172/JCI13785
Hakonen E, Ustinov J, Eizirik DL, Sariola H, Miettinen PJ, Otonkoski T (2014) In vivo activation of the PI3K-Akt pathway in mouse beta cells by the EGFR mutation L858R protects against diabetes. Diabetologia 57:970–979. https://doi.org/10.1007/s00125-014-3175-2
Stiles BL, Kuralwalla-Martinez C, Guo W, Gregorian C, Wang Y, Tian J, Magnuson MA, Wu H (2006) Selective deletion of Pten in pancreatic β cells leads to increased islet mass and resistance to STZ-induced diabetes. Mol Cell Biol 26:2772–2781. https://doi.org/10.1128/MCB.26.7.2772-2781.2006
Shuai H, Zhang J, Zhang J, Xie J, Zhang M, Yu Y, Zhang L (2011) Erythropoietin protects pancreatic beta-cell line NIT-1 cells against cytokine-induced apoptosis via phosphatidylinositol 3-kinase/Akt signaling. Endocr Res 36:25–34. https://doi.org/10.3109/07435800.2010.534753
Gui S, Yuan G, Wang L, Zhou L, Xue Y, Yu Y, Zhang J, Zhang M, Yang Y, Wang DW (2013) Wnt3a regulates proliferation, apoptosis and function of pancreatic NIT-1 beta cells via activation of IRS2/PI3K signaling. J Cell Biochem 114:1488–1497. https://doi.org/10.1002/jcb.24490
Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J (1994) PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature 370:71–75. https://doi.org/10.1038/370071a0
De Santis G, Miotti S, Mazzi M, Canevari S, Tomassetti A (2009) E-cadherin directly contributes to PI3K/AKT activation by engaging the PI3K-p85 regulatory subunit to adherens junctions of ovarian carcinoma cells. Oncogene 28:1206–1217. https://doi.org/10.1038/onc.2008.470
Nacerddine K, Beaudry JB, Ginjala V, Westerman B, Mattiroli F, Song JY, van der Poel H, Ponz OB, Pritchard C, Cornelissen-Steijger P et al (2012) Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer. J Clin Invest 122:1920–1932. https://doi.org/10.1172/jci57477
Di L (2015) Strategic approaches to optimizing peptide ADME properties. AAPS J 17:134–143. https://doi.org/10.1208/s12248-014-9687-3
Hamaguchi K, Gaskins HR, Leiter EH (1991) NIT-1, a pancreatic β-cell line established from a transgenic NOD/Lt mouse. Diabetes 40:842–849. https://doi.org/10.2337/diab.40.7.842
Petit PX, Gendron M-C, Schrantz N, Métivier D, Kroemer G, Maciorowska Z, Sureau F, Koester S (2001) Oxidation of pyridine nucleotides during Fas-and ceramide-induced apoptosis in Jurkat cells: correlation with changes in mitochondria, glutathione depletion, intracellular acidification and caspase 3 activation. Biochem J 353:357–367
Kauffmann-Zeh A, Rodriguez-Viciana P, Ulrich E, Gilbert C, Coffer P, Downward J, Evan G (1997) Suppression of c-Myc-induced apoptosis by Ras signalling through PI (3) K and PKB. Nature 385:544–548. https://doi.org/10.1038/385544a0
Khwaja A, Rodriguez-Viciana P, Wennström S, Warne PH, Downward J (1997) Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J 16:2783–2793. https://doi.org/10.1093/emboj/16.10.2783
Kim S-J, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH (2005) Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic β-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 280:22297–22307. https://doi.org/10.1074/jbc.M500540200
Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ (2002) Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic β-cells (INS-1). J Biol Chem 277:49676–49684. https://doi.org/10.1074/jbc.M208756200
Størling J, Binzer J, Andersson AK, Züllig R, Tonnesen M, Lehmann R, Spinas G, Sandler S, Billestrup N, Mandrup-Poulsen T (2005) Nitric oxide contributes to cytokine-induced apoptosis in pancreatic beta cells via potentiation of JNK activity and inhibition of Akt. Diabetologia 48:2039–2050. https://doi.org/10.1007/s00125-005-1912-2
Tuttle RL, Gill NS, Pugh W, Lee JP, Koeberlein B, Furth EE, Polonsky KS, Naji A, Birnbaum MJ (2001) Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med 7:1133–1137. https://doi.org/10.1038/nm1001-1133
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N (2001) Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev 15:1406–1418. https://doi.org/10.1101/gad.889901
Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM (2004) Akt stimulates aerobic glycolysis in cancer cells. Can Res 64:3892–3899. https://doi.org/10.1158/0008-5472.CAN-03-2904
Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB (2003) Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol 23:7315–7328. https://doi.org/10.1128/mcb.23.20.7315-7328.2003
Hoxhaj G, Manning BD (2020) The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer 20:74–88. https://doi.org/10.1038/s41568-019-0216-7
Huang T, Wang L, Liu D, Li P, Xiong H, Zhuang L, Sun L, Yuan X, Qiu H (2017) FGF7/FGFR2 signal promotes invasion and migration in human gastric cancer through upregulation of thrombospondin-1. Int J Oncol 50:1501–1512. https://doi.org/10.3892/ijo.2017.3927
Cunha DA, Cito M, Carlsson P-O, Vanderwinden J-M, Molkentin JD, Bugliani M, Marchetti P, Eizirik DL, Cnop M (2016) Thrombospondin 1 protects pancreatic β-cells from lipotoxicity via the PERK–NRF2 pathway. Cell Death Differ 23:1995–2006. https://doi.org/10.1038/cdd.2016.89
Tonelli C, Chio IIC, Tuveson DA (2018) Transcriptional regulation by Nrf2. Antioxid Redox Signal 29:1727–1745. https://doi.org/10.1089/ars.2017.7342
Zhang X, Tang N, Hadden TJ, Rishi AK (2011) Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta Mol Cell Res 1813:1978–1986. https://doi.org/10.1016/j.bbamcr.2011.03.010
Tang ED, Nuñez G, Barr FG, Guan K-L (1999) Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem 274:16741–16746. https://doi.org/10.1074/jbc.274.24.16741
Cornu M, Thorens B (2009) GLP-1 protects β-cells against apoptosis by enhancing the activity of an IGF-2/IGF1-receptor autocrine loop. Islets 1:280–282. https://doi.org/10.4161/isl.1.3.9932
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868. https://doi.org/10.1016/s0092-8674(00)80595-4
Zurawek M, Fichna M, Fichna P, Czainska M, Rozwadowska N (2020) Upregulation of FOXO3 in new-onset type 1 diabetes mellitus. J Immunol Res 2020. https://doi.org/10.1155/2020/9484015
Hunter RW, Hughey CC, Lantier L, Sundelin EI, Peggie M, Zeqiraj E, Sicheri F, Jessen N, Wasserman DH, Sakamoto K (2018) Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat Med 24:1395–1406. https://doi.org/10.1038/s41591-018-0159-7
Brereton MF, Rohm M, Shimomura K, Holland C, Tornovsky-Babeay S, Dadon D, Iberl M, Chibalina MV, Lee S, Glaser B et al (2016) Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat Commun 7:13496. https://doi.org/10.1038/ncomms13496
Volat FE, Pointud JC, Pastel E, Morio B, Sion B, Hamard G, Guichardant M, Colas R, Lefrançois-Martinez AM, Martinez A (2012) Depressed levels of prostaglandin F2α in mice lacking Akr1b7 increase basal adiposity and predispose to diet-induced obesity. Diabetes 61:2796–2806. https://doi.org/10.2337/db11-1297
Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R (1997) Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem 272:13088–13093. https://doi.org/10.1074/jbc.272.20.13088
Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J (2015) Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43:304–317. https://doi.org/10.1016/j.immuni.2015.07.013
Sun J, Xu M, Ortsater H, Lundeberg E, Juntti-Berggren L, Chen YQ, Haeggstrom JZ, Gudmundsson GH, Diana J, Agerberth B (2016) Cathelicidins positively regulate pancreatic beta-cell functions. FASEB J 30:884–894. https://doi.org/10.1096/fj.15-275826
Wang X, Chen L, Zhao X, Xiao L, Yi S, Kong Y, Jiang Y, Zhang J (2020) A cathelicidin-related antimicrobial peptide suppresses cardiac hypertrophy induced by pressure overload by regulating IGFR1/PI3K/AKT and TLR9/AMPKα. Cell Death Dis 11:1–15. https://doi.org/10.1038/s41419-020-2296-4
Underwood CR, Garibay P, Knudsen LB, Hastrup S, Peters GH, Rudolph R, Reedtz-Runge S (2010) Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J Biol Chem 285:723–730. https://doi.org/10.1074/jbc.M109.033829
Oddo A, Mortensen S, Thøgersen H, De Maria L, Hennen S, McGuire JN, Kofoed J, Linderoth L, Reedtz-Runge S (2018) α-Helix or β-turn? An investigation into N-terminally constrained analogues of glucagon-like peptide 1 (GLP-1) and exendin-4. Biochemistry 57:4148–4154. https://doi.org/10.1021/acs.biochem.8b00105
Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE (2004) Helminth parasites–masters of regulation. Immunol Rev 201:89–116. https://doi.org/10.1111/j.0105-2896.2004.00191.x
Allen JE, Maizels RM (2011) Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11:375–388. https://doi.org/10.1038/nri2992
Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW (1999) The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med 189:347–358. https://doi.org/10.1084/jem.189.2.347
Alvarado R, To J, Lund ME, Pinar A, Mansell A, Robinson MW, O’Brien BA, Dalton JP, Donnelly S (2017) The immune modulatory peptide FhHDM-1 secreted by the helminth Fasciola hepatica prevents NLRP3 inflammasome activation by inhibiting endolysosomal acidification in macrophages. FASEB J 31:85–95. https://doi.org/10.1096/fj.201500093R
Acknowledgements
We thank the UTS Microbial Imaging Facility and director Dr L. Cole for providing technical training for the Zeiss slide scanner and A. Zebib and E. van Dijk for assisting in the sectioning of mouse pancreata. We would also like to acknowledge the guidance provided by Alison Ricafrente and Dr Alen Faiz for the EdgeR analysis of the transcriptome data. Commentary and feedback on the final manuscript from Prof. John Dalton and Prof. Bruce Milthorpe were gratefully received.
Funding
This work was funded by a National Health and Medical Research Council project grant (APP1087341), a JDRF Beta Cell Regeneration Innovative grant (1-INO-2019-785-S-B) and a Royal Society research grant (RG2012R2).
Author information
Authors and Affiliations
Contributions
I.C. and T.Y.M. performed the experiments; I.C., M.L., J.T., N.B., M.W.R., J.S. and S.D. performed data analysis; I.C., S.D. and B.O.B. designed the study and all authors contributed to the discussion and manuscript preparation.
Corresponding author
Ethics declarations
Ethics approval
All animal work was conducted in accordance with the approved guidelines of the University of Technology Sydney Animal Care and Ethics Committee (protocols ETH17-1226 and ETH19-4082).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Camaya, I., Mok, T.Y., Lund, M. et al. The parasite-derived peptide FhHDM-1 activates the PI3K/Akt pathway to prevent cytokine-induced apoptosis of β-cells. J Mol Med 99, 1605–1621 (2021). https://doi.org/10.1007/s00109-021-02122-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-021-02122-x