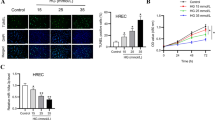
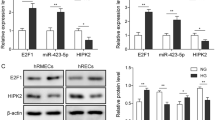
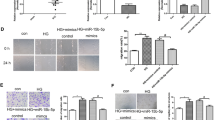
Abstract
Background
Diabetic retinopathy (DR), currently considered as a neurovascular disease, has become the major cause of blindness. More and more scholars believe that DR is no longer just a kind of microvascular disease, but accompanied by retinal neurodegenerative changes. Intravitreal injection of anti-vascular endothelial growth factor (VEGF) drugs is a classic treatment for DR; however, anti-VEGF drugs can exacerbate fibrosis and eventually lead to retinal detachment. The aim of this study was to explore the pathogenesis of DR and identified new treatments that can provide dual-target intervention for angiogenesis and fibrosis.
Methods
We explored changes in gene expression in high glucose–induced vascular endothelial cells using RNA sequencing (RNA-seq) technology. We identified bone morphogenetic protein 4 (BMP4) and SMAD family member 9 (SMAD9) among 449 differentially expressed genes from RNA-seq data and confirmed the expression of these two genes in the blood of diabetes patients by RT-PCR and in streptozotocin-induced rat retinas by RT-PCR, immunofluorescence, and western blot. Moreover, considering that DR is a multifactorial and multicellular disease, we used hydrogen peroxide (H2O2), advanced glycation end products (AGEs), CoCl2, 4-hydroxynonenal (4-HNE), and hypoxia to induce three human retinal cell types (Müller, retinal pigment epithelium, and human retinal capillary endothelial cells) to simulate the pathogenesis of DR, and MTT experiment, scratch experiment, Transwell experiment, and lumen formation experiment were used to test whether the model was successfully established. Then, we verified the overexpression of these two genes in the cell models by RT-PCR, immunofluorescence, and western blot. We further tested the effects of BMP4 on retinal cells. We use BMP4 to stimulate retinal cells and observe the effect of BMP4 on retinal cells by MTT experiment, scratch experiment, and RT-PCR.
Results
The results demonstrated that BMP4 and SMAD9 were highly expressed in both in vivo and in vitro models, while BMP4 could significantly upregulate the expression of SMAD9 and promote the expression of VEGF and fibrosis factors.
Conclusions
This study is the first to analyze the mechanism by which high glucose levels affect retinal vascular endothelial cells through RNA transcriptome sequencing and indicates that BMP4 may be a potential target for the dual-target treatment (anti-VEGF and anti-fibrosis) of DR.
Key messages
• High-glucose effect on vascular endothelial cell was analyzed by RNA-seq.
• KEGG analysis revealed enrichment of TGF-beta signaling pathway.
• SMAD9 and BMP4 expression was upregulated in all samples.
• Dual-target therapy of PDR by antagonizing BMP4.






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DR:
-
diabetic retinopathy
- GO:
-
Gene Ontology (GO)
- HRCECs:
-
human retinal capillary endothelial cells
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NEB:
-
New England Biolabs (NEB)
- nr:
-
nonredundant
- PFA:
-
paraformaldehyde
- RNA-seq:
-
sequencing
- RT-qPCR:
-
reverse transcription quantitative PCR
- SD:
-
Sprague Dawley
- STZ:
-
streptozotocin
References
Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR (2017) Diabetic retinopathy: breaking the barrier. Pathophysiology 24:229–241
Gangwani RA, Lian JX, McGhee SM, Wong D, Li KK (2016) Diabetic retinopathy screening: global and local perspective. Hong Kong Med J 22:486–495
Simo R, Hernandez C, European Consortium for the Early Treatment of Diabetic Retinopathy (2014) Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol Metab 25:23–33
Gardner TW, Davila JR (2017) The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 255:1–6
Hernandez C, Simo-Servat A, Bogdanov P, Simo R (2017) Diabetic retinopathy: new therapeutic perspectives based on pathogenic mechanisms. J Endocrinol Investig 40:925–935
Dong L, Nian H, Shao Y, Zhang Y, Li Q, Yi Y, Tian F, Li W, Zhang H, Zhang X, Wang F, Li X (2015) PTB-associated splicing factor inhibits IGF-1-induced VEGF upregulation in a mouse model of oxygen-induced retinopathy. 360:233–243
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R (2016) Diabetic retinopathy. Nat Rev Dis Primers 2:16012
Xing X, Huang L, Lv Y, Liu X, Su R, Li X, Dong L (2019) DL-3-n-butylphthalide protected retinal Müller cells dysfunction from oxidative stress. Curr Eye Res 44:1112–1120
Shao Y, Dong L, Takahashi Y, Chen J, Liu X, Chen Q, Ma J, Li X (2019) MiRNA-451a regulates RPE function through promoting mitochondrial function in proliferative diabetic retinopathy. Am J Physiol Endocrinol Metab 316:E443–E452
Shao Y, Chen J, Dong L, He X, Cheng R, Zhou K, Liu J, Qiu F, Li X, Ma J (2019) A protective effect of PPARα in endothelial progenitor cells through regulating metabolism. Diabetes 68:12131–12142
Shao Y, Dong L, Zhang Y, Liu H, Hu B, Liu J, Li X (2015) Surgical induced astigmatism correlated with corneal pachymetry and intraocular pressure: transconjunctival sutureless 23-gauge versus 20-gauge sutured vitrectomy in diabetes mellitus. Int J Ophthalmol 8:528–533
Bressler NM, Beaulieu WT, Bressler SB, Glassman AR, Melia BM, Jampol LM, Jhaveri CD, Salehi-Had H, Velez G, Sun JK, DRCR Retina Network (2019) Anti-vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: pooled Analysis of five DRCR retina network randomized clinical trials. Retina
Shao Y, Chen J, Freeman W, Dong L, Zhang Z, Xu M, Qiu F, Du Y, Liu J, Li X, Ma J (2019) Canonical Wnt signaling promotes neovascularization through determination of endothelial progenitor cell fate via metabolic profile regulation. Stem Cells 37:1331–1343
Klaassen I, van Geest RJ, Kuiper EJ, van Noorden CJ, Schlingemann RO (2015) The role of CTGF in diabetic retinopathy. Exp Eye Res 133:37–48
Yang H, Huang Y, Chen X, Liu J, Lu Y, Bu L, Xia L, Xiao W, Chen M, Nie Q, Liu Z (2010) The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-beta(2), elucidated by treatment with CTGFsiRNA. Acta Ophthalmol 88:652–659
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Mantione KJ, Kream RM, Kuzelova H, Ptacek R, Raboch J, Samuel JM, Stefano GB (2014) Comparing bioinformatic gene expression profiling methods: microarray and RNA-Seq. Med Sci Monit Basic Res 20:138–142
Stewart MW, Browning DJ, Landers MB (2018) Current management of diabetic tractional retinal detachments. Indian J Ophthalmol 66:1751–1762
Osaadon P, Fagan XJ, Lifshitz T, Levy J (2014) A review of anti-VEGF agents for proliferative diabetic retinopathy. Eye (Lond) 28:510–520
Li W, Dong L, Ma M, Hu B, Lu Z, Liu X, Liu J, Li X (2016) Preliminary in vitro and in vivo assessment of a new targeted inhibitor for choroidal neovascularization in age-related macular degeneration. Drug Des Devel Ther 10:3415–3423
Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO (2008) The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One 3:e2675
Schmitt JM, Hwang K, Winn SR, Hollinger JO (1999) Bone morphogenetic proteins: an update on basic biology and clinical relevance. J Orthop Res 17:269–278
Vogt RR, Unda R, Yeh LC, Vidro EK, Lee JC, Tsin AT (2006) Bone morphogenetic protein-4 enhances vascular endothelial growth factor secretion by human retinal pigment epithelial cells. J Cell Biochem 98:1196–1202
Fischer AJ, Schmidt M, Omar G, Reh TA (2004) BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci 27:531–542
Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK (2005) Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res 65:448–456
He C, Chen X (2005) Transcription regulation of the vegf gene by the BMP/Smad pathway in the angioblast of zebrafish embryos. Biochem Biophys Res Commun 329:324–330
Frank D, Johnson J, de Caestecker M (2005) Bone morphogenetic protein 4 promotes vascular remodeling in hypoxic pulmonary hypertension. Chest 128:590S–591S
Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A (2011) Bone morphogenetic proteins: a critical review. Cell Signal 23:609–620
Miyazono K, Kamiya Y, Morikawa M (2010) Bone morphogenetic protein receptors and signal transduction. J Biochem 147:35–51
Dong L, Zhang X, Fu X, Zhang X, Gao X, Zhu M, Wang X, Yang Z, Jensen ON, Saarikettu J, Yao Z, Silvennoinen O, Yang J (2011) PTB-associated splicing factor (PSF) functions as a repressor of STAT6-mediated Ig epsilon gene transcription by recruitment of HDAC1. J Biol Chem 286:3451–3459
Tian F, Dong L, Zhou Y, Shao Y, Li W, Zhang H, Wang F (2014) Rapamycin-induced apoptosis in HGF-stimulated lens epithelial cells by AKT/mTOR, ERK and JAK2/STAT3 pathways. Int J Mol Sci 15:13833–13848
Cheng KH, Ponte JF, Thiagalingam S (2004) Elucidation of epigenetic inactivation of SMAD8 in cancer using targeted expressed gene display. Cancer Res 64:1639–1646
Katakawa Y, Funaba M, Murakami M (2016) Smad8/9 is regulated through the BMP pathway. J Cell Biochem 117:1788–1796
Koli K, Myllarniemi M, Vuorinen K, Salmenkivi K, Ryynanen MJ, Kinnula VL, Keski-Oja J (2006) Bone morphogenetic protein-4 inhibitor gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol 169:61–71
Mugisho OO, Green CR, Squirrell DM, Bould S, Danesh-Meyer HV, Zhang J, Acosta ML, Rupenthal ID (2019) Connexin43 hemichannel block protects against the development of diabetic retinopathy signs in a mouse model of the disease. J Mol Med (Berl) 97:215–229
Zeiner J, Loukovaara S, Losenkova K, Zuccarini M, Korhonen AM, Lehti K, Kauppinen A, Kaarniranta K, Müller CE, Jalkanen S, Yegutkin GG (2019) Soluble and membrane-bound adenylate kinase and nucleotidases augment ATP-mediated inflammation in diabetic retinopathy eyes with vitreous hemorrhage. J Mol Med (Berl) 97:341–354
Ellis EA, Guberski DL, Somogyi-Mann M, Grant MB (2000) Increased H2O2, vascular endothelial growth factor and receptors in the retina of the BBZ/Wor diabetic rat. Free Radic Biol Med 28:91–101
Osera C, Martindale JL, Amadio M, Kim J, Yang X, Moad CA, Indig FE, Govoni S, Abdelmohsen K, Gorospe M, Pascale A (2015) Induction of VEGFA mRNA translation by CoCl2 mediated by HuR. RNA Biol 12:1121–1130
Gu L, Xu H, Zhang C, Yang Q, Zhang L, Zhang J (2019) Time-dependent changes in hypoxia- and gliosis-related factors in experimental diabetic retinopathy. Eye (Lond) 33:600–609
Lei XW, Li Q, Zhang JZ, Zhang YM, Liu Y, Yang KH (2019) The protective roles of folic acid in preventing diabetic retinopathy are potentially associated with suppressions on angiogenesis, inflammation, and oxidative stress. Ophthalmic Res 62:80–92
Stitt AW (2010) AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci 51:4867–4874
Hu B, Zhang Y, Zeng Q, Han Q, Zhang L, Liu M, Li X (2014) Intravitreal injection of ranibizumab and CTGF shRNA improves retinal gene expression and microvessel ultrastructure in a rodent model of diabetes. Int J Mol Sci 15:1606–1624
Acknowledgments
The authors thank all clinical laboratory colleagues for their assistance with the regular blood sample collection. We are also thankful for the support and assistance from the Tianjin Medical University Eye Institute Biobank.
Funding
This study was supported by the National Natural Science Funds (81570872), China; National Natural Science Foundation of China (81670875), Tianjin Municipal Health and Family Planning Commission young medical talents project, Tianjin, China; Tianjin Medical University Eye Hospital young innovative talents project (YDYYRCXM-C2018-02), Tianjin, China; Tianjin Education Commission research project (2018KJ063), Tianjin, China; Bethune Merck Diabetes Research Foundation; Tianjin clinical key discipline construction project (TJLCZDXKM010), Tianjin, China; and Tianjin Medical University Eye Hospital clinical research fund (2016LCKY005).
Author information
Authors and Affiliations
Contributions
Li Jiedong designed the research; Xiaorong Li provided advice and guidance; Zhe Zhang and Xun Liu performed the research; Zhe Zhang and Juping Liu wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they no conflicts of interests.
Ethical approval and consent to participate
All human studies have been approved by the Ethics Committee of Tianjin Medical University Eye Hospital, and animal experimentation conforms to protocols were approved by the Animal Ethical and Welfare Committee of Tianjin Medical University Eye Institute.
Consent for publication
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dong Lijie and Zhang Zhe as the co-first authors, contributed equally to this manuscript.
Supplementary Information
Supplementary Figure 1
(PNG 4226 kb)
Supplementary Figure 2
(PNG 2292 kb)
Supplementary Figure 3
(PNG 2201 kb)
Supplementary Figure 4
(PNG 3385 kb)
Supplementary Figure 5
(PNG 1986 kb)
Supplementary Figure 6
(PNG 3176 kb)
Supplementary Figure 7
Hypothesis: Signal pathway of BMP4 and SMAD9 act on the development of DR. (PNG 1042 kb)
Rights and permissions
About this article
Cite this article
Dong, L., Zhang, Z., Liu, X. et al. RNA sequencing reveals BMP4 as a basis for the dual-target treatment of diabetic retinopathy. J Mol Med 99, 225–240 (2021). https://doi.org/10.1007/s00109-020-01995-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01995-8