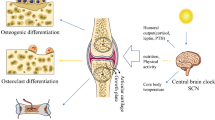
Abstract
The 24-h rhythm of behavioral and physiological processes is a typical biological phenomenon regulated by a group of circadian rhythm genes. Dysfunction of the circadian rhythm can cause a wide range of problems, such as cancer and metabolic diseases. In recent decades, increased understanding of the roles of circadian rhythm genes in the bone remodeling process have been documented, including osteoblastic bone formation, osteoclastic bone resorption, and osteoblast/osteoclast communication. A timely review of the current findings may help to facilitate the new field of circadian rhythmic bone remodeling research. Targeted pharmacological modulation of circadian rhythm genes is a possible therapeutic approach through which to overcome bone remodeling problems in the future.


Similar content being viewed by others
References
Zofkova I (2015) Bone tissue as a systemic endocrine regulator. Physiol Res 64(4):439–445
Guerrini MM, Takayanagi H (2014) The immune system, bone and RANKL. Arch Biochem Biophys 561:118–123
Matsumoto T (2011) Progress and perspectives in bone research. Nihon Rinsho 69(7):1175–1180
Sun W, Shi Y, Lee WC, Lee SY, Long F (2016) Rictor is required for optimal bone accrual in response to anti-sclerostin therapy in the mouse. Bone 85:1–8
Yuan FL, Wu QY, Miao ZN, Xu MH, Xu RS, Jiang DL, Ye JX, Chen FH, Zhao MD, Wang HJ, Li X (2018) Osteoclast-derived extracellular vesicles: novel regulators of osteoclastogenesis and osteoclast-osteoblasts communication in bone remodeling. Front Physiol 9:628
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94(1):25–34
Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015:421746
Jimi E (2017) The role of osteoclastic bone resorption on bone remodeling. Clin Calcium 27(12):1689–1695
Li J, Bao Q, Chen S, Liu H, Feng J, Qin H, Li A, Liu D, Shen Y, Zhao Y, Zong Z (2017) Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/beta-catenin signaling in mice. J Orthop Res 35(4):812–819
Wu QY, Li X, Miao ZN, Ye JX, Wang B, Zhang F, Xu RS, Jiang DL, Zhao MD, Yuan FL (2018) Long noncoding RNAs: a new regulatory code for osteoporosis. Front Endocrinol (Lausanne) 9:587
Sozen T, Ozisik L, Basaran NC (2017) An overview and management of osteoporosis. Eur J Rheumatol 4(1):46–56
Reginster JY, Burlet N (2006) Osteoporosis: a still increasing prevalence. Bone 38(2 Suppl 1):S4–S9
Albayrak I, Aydogmus M, Ozerbil OM, Levendoglu F (2016) The association between bone mineral density, quality of life, quality of sleep and fatigue. Acta Clin Belg 71(2):92–98
Cunningham TD, Di Pace BS (2015) Is self-reported sleep duration associated with osteoporosis? Data from a 4-year aggregated analysis from the National Health and Nutrition Examination Survey. J Am Geriatr Soc 63(7):1401–1406
Swanson CM, Shea SA, Stone KL, Cauley JA, Rosen CJ, Redline S, Karsenty G, Orwoll ES (2015) Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep. J Bone Miner Res 30(2):199–211
Tan E, Scott EM (2014) Circadian rhythms, insulin action, and glucose homeostasis. Curr Opin Clin Nutr Metab Care 17(4):343–348
Green CB, Takahashi JS, Bass J (2008) The meter of metabolism. Cell 134(5):728–742
de Crombrugghe B (2005) Osteoblasts clock in for their day job. Cell 122(5):651–653
Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G (2005) The molecular clock mediates leptin-regulated bone formation. Cell 122(5):803–815
Iimura T, Nakane A, Sugiyama M, Sato H, Makino Y, Watanabe T, Takagi Y, Numano R, Yamaguchi A (2012) A fluorescence spotlight on the clockwork development and metabolism of bone. J Bone Miner Metab 30(3):254–269
Lieben L (2016) Bone: the circadian clock controls bone remodelling. Nat Rev Rheumatol 12(3):132–133
Dudek M, Meng QJ (2014) Running on time: the role of circadian clocks in the musculoskeletal system. Biochem J 463(1):1–8
Ledger S, Strayer C, Ashton F, Kay SA, Putterill J (2001) Analysis of the function of two circadian-regulated CONSTANS-LIKE genes. Plant J 26(1):15–22
Pellegrini GG, Gonzales Chaves MM, Fajardo MA, Ponce GM, Toyos GI, Lifshitz F, Friedman SM, Zeni SN (2012) Salivary bone turnover markers in healthy pre- and postmenopausal women: daily and seasonal rhythm. Clin Oral Investig 16(2):651–657
Shao P, Ohtsuka-Isoya M, Shinoda H (2003) Circadian rhythms in serum bone markers and their relation to the effect of etidronate in rats. Chronobiol Int 20(2):325–336
Fujihara Y, Kondo H, Noguchi T, Togari A (2014) Glucocorticoids mediate circadian timing in peripheral osteoclasts resulting in the circadian expression rhythm of osteoclast-related genes. Bone 61:1–9
Rosenwasser AM, Turek FW (2015) Neurobiology of circadian rhythm regulation. Sleep Med Clin 10(4):403–412
Reddy AB, Rey G (2014) Metabolic and nontranscriptional circadian clocks: eukaryotes. Annu Rev Biochem 83:165–189
Brown SA, Kowalska E, Dallmann R (2012) (Re)inventing the circadian feedback loop. Dev Cell 22(3):477–487
Huang N, Chelliah Y, Shan Y, Taylor CA, Yoo SH, Partch C, Green CB, Zhang H, Takahashi JS (2012) Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science 337(6091):189–194
Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280(5369):1564–1569
Yagita K, Yamaguchi S, Tamanini F, van Der Horst GT, Hoeijmakers JH, Yasui A, Loros JJ, Dunlap JC, Okamura H (2000) Dimerization and nuclear entry of mPER proteins in mammalian cells. Genes Dev 14(11):1353–1363
Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM (1999) mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98(2):193–205
van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, Bootsma D, Hoeijmakers JH, Yasui A (1999) Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398(6728):627–630
Yu W, Nomura M, Ikeda M (2002) Interactivating feedback loops within the mammalian clock: BMAL1 is negatively autoregulated and upregulated by CRY1, CRY2, and PER2. Biochem Biophys Res Commun 290(3):933–941
Crumbley C, Wang Y, Kojetin DJ, Burris TP (2010) Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J Biol Chem 285(46):35386–35392
Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U (2002) The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110(2):251–260
Guillaumond F, Dardente H, Giguere V, Cermakian N (2005) Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythm 20(5):391–403
Buhr ED, Takahashi JS (2013) Molecular components of the mammalian circadian clock. Handb Exp Pharmacol 217:3–27
Robinson I, Reddy AB (2014) Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett 588(15):2477–2483
Takarada T, Xu C, Ochi H, Nakazato R, Yamada D, Nakamura S, Kodama A, Shimba S, Mieda M, Fukasawa K, Ozaki K, Iezaki T, Fujikawa K, Yoneda Y, Numano R, Hida A, Tei H, Takeda S, Hinoi E (2017) Bone resorption is regulated by circadian clock in osteoblasts. J Bone Miner Res 32(4):872–881
Samsa WE, Vasanji A, Midura RJ, Kondratov RV (2016) Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 84:194–203
Yuan G, Hua B, Yang Y, Xu L, Cai T, Sun N, Yan Z, Lu C, Qian R (2017) The circadian gene clock regulates bone formation via PDIA3. J Bone Miner Res 32(4):861–871
Maronde E, Schilling AF, Seitz S, Schinke T, Schmutz I, van der Horst G, Amling M, Albrecht U (2010) The clock genes period 2 and Cryptochrome 2 differentially balance bone formation. PLoS One 5(7):e11527. https://doi.org/10.1371/journal.pone.0011527
He Y, Chen Y, Zhao Q, Tan Z (2013) Roles of brain and muscle ARNT-like 1 and Wnt antagonist Dkk1 during osteogenesis of bone marrow stromal cells. Cell Prolif 46(6):644–653
Lin F, Chen Y, Li X, Zhao Q, Tan Z (2013) Over-expression of circadian clock gene Bmal1 affects proliferation and the canonical Wnt pathway in NIH-3T3 cells. Cell Biochem Funct 31(2):166–172
Zhang H, Lu W, Zhao Y, Rong P, Cao R, Gu W, Xiao J, Miao D, Lappe J, Recker R, Xiao GG (2011) Adipocytes derived from human bone marrow mesenchymal stem cells exert inhibitory effects on osteoblastogenesis. Curr Mol Med 11(6):489–502
Patel SA, Velingkaar NS, Kondratov RV (2014) Transcriptional control of antioxidant defense by the circadian clock. Antioxid Redox Signal 20(18):2997–3006
Jouffe C, Cretenet G, Symul L, Martin E, Atger F, Naef F, Gachon F (2013) The circadian clock coordinates ribosome biogenesis. PLoS Biol 11(1):e1001455. https://doi.org/10.1371/journal.pbio.1001455
Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Guttler T, Davis F, Asara JM, Sahin M (2015) The circadian protein BMAL1 regulates translation in response to S6K1-mediated phosphorylation. Cell 161(5):1138–1151
Hirai T, Tanaka K, Togari A (2014) Beta-adrenergic receptor signaling regulates Ptgs2 by driving circadian gene expression in osteoblasts. J Cell Sci 127(Pt 17):3711–3719
Xu C, Ochi H, Fukuda T, Sato S, Sunamura S, Takarada T, Hinoi E, Okawa A, Takeda S (2016) Circadian clock regulates bone resorption in mice. J Bone Miner Res 31(7):1344–1355
Aoyama S, Shibata S (2017) The role of circadian rhythms in muscular and osseous physiology and their regulation by nutrition and exercise. Front Neurosci 11:63. https://doi.org/10.3389/fnins.2017.00063
Stashi E, Lanz RB, Mao J, Michailidis G, Zhu B, Kettner NM, Putluri N, Reineke EL, Reineke LC, Dasgupta S, Dean A, Stevenson CR, Sivasubramanian N, Sreekumar A, Demayo F, York B, Fu L, O'Malley BW (2014) SRC-2 is an essential coactivator for orchestrating metabolism and circadian rhythm. Cell Rep 6(4):633–645
Guntur AR, Kawai M, Le P, Bouxsein ML, Bornstein S, Green CB, Rosen CJ (2011) An essential role for the circadian-regulated gene Nocturnin in osteogenesis: the importance of local timekeeping in skeletal homeostasis. Ann N Y Acad Sci 1237:58–63
Kim M, de la Pena JB, Cheong JH, Kim HJ (2017) Neurobiological functions of the period circadian clock 2 gene, Per2. Biomol Ther (Seoul) 26:358–367
Zhao B (2017) TNF and bone remodeling. Curr Osteoporos Rep 15(3):126–134
Bellet MM, Nakahata Y, Boudjelal M, Watts E, Mossakowska DE, Edwards KA, Cervantes M, Astarita G, Loh C, Ellis JL, Vlasuk GP, Sassone-Corsi P (2013) Pharmacological modulation of circadian rhythms by synthetic activators of the deacetylase SIRT1. Proc Natl Acad Sci U S A 110(9):3333–3338
Funding
This study was supported by the Natural Science Foundation of China (81270011; 81472125), the Natural Science Foundation of Jiangsu Province (Grant BK20151114), Foundation of Traditional Chinese Medicine of Jiangsu Province (YB201578), and Fundamental Research Funds for Medical Innovation Center of Health and Family Planning Commission of Wuxi (CXTD006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wu, QY., Wang, J., Tong, X. et al. Emerging role of circadian rhythm in bone remodeling. J Mol Med 97, 19–24 (2019). https://doi.org/10.1007/s00109-018-1723-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-018-1723-9