Abstract
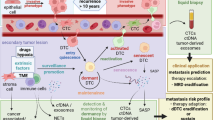
Disseminated tumor cells (DTCs) are detected early in the disease process in prostate cancer (PCa) patients and can persist after radical prostatectomy. DTCs can remain dormant in patients with no evidence of disease for a prolonged period of time only to recur 10 or more years later. Recent advances in single-cell genomics and transcriptomics have provided much needed insight into DTC biology and cancer dormancy in patients. With the development of new in vitro and preclinical models, researchers recapitulate the clinical events in patients and therefore allow further elucidation of the molecular mechanisms underlying cancer dormancy and escape. In this review, we explore novel ideas on the detection, heterogeneous transcriptomic profiles, molecular and cellular mechanisms of dormancy, and potential mechanisms underlying dormancy escape by DTCs. As such, there is hope that identifying and targeting novel dormancy-associated pathways in patients with residual disease will have significant clinical implications for the treatment of PCa patients in the future.

Similar content being viewed by others
References
Weckermann D, Muller P, Wawroschek F, Krawczak G, Riethmuller G, Schlimok G (1999) Micrometastases of bone marrow in localized prostate cancer: correlation with established risk factors. J Clin Oncol 17:3438–3443
Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL (2009) Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res 15:677–683
Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H (2000) Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol 164:101–105
Loeb S, Feng Z, Ross A, Trock BJ, Humphreys EB, Walsh PC (2011) Can we stop prostate specific antigen testing 10 years after radical prostatectomy? J Urol 186:500–505
Ahove DA, Hoffman KE, Hu JC, Choueiri TK, D'Amico AV, Nguyen PL (2010) Which patients with undetectable PSA levels 5 years after radical prostatectomy are still at risk of recurrence?--implications for a risk-adapted follow-up strategy. Urology 76:1201–1205
Chery L, Lam HM, Coleman I, Lakely B, Coleman R, Larson S, Aguirre-Ghiso JA, Xia J, Gulati R, Nelson PS et al (2014) Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget 5:9939–9951
Lilleby W, Stensvold A, Mills IG, Nesland JM (2013) Disseminated tumor cells and their prognostic significance in nonmetastatic prostate cancer patients. Int J Cancer 133:149–155
Todenhofer T, Hennenlotter J, Faber F, Wallwiener D, Schilling D, Kuhs U, Aufderklamm S, Bier S, Mischinger J, Gakis G et al (2015) Significance of apoptotic and non-apoptotic disseminated tumor cells in the bone marrow of patients with clinically localized prostate cancer. Prostate 75:637–645
Wood DP Jr, Banerjee M (1997) Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J Clin Oncol 15:3451–3457
Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL (2003) Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology 61:277–281
Pfitzenmaier J, Ellis WJ, Hawley S, Arfman EW, Klein JR, Lange PH, Vessella RL (2007) The detection and isolation of viable prostate-specific antigen positive epithelial cells by enrichment: a comparison to standard prostate-specific antigen reverse transcriptase polymerase chain reaction and its clinical relevance in prostate cancer. Urol Oncol 25:214–220
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB (1999) Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res 59:3192–3198
Weckermann D, Muller P, Wawroschek F, Harzmann R, Riethmuller G, Schlimok G (2001) Disseminated cytokeratin positive tumor cells in the bone marrow of patients with prostate cancer: detection and prognostic value. J Urol 166:699–703
Klein CA, Schmidt-Kittler O, Schardt JA, Pantel K, Speicher MR, Riethmuller G (1999) Comparative genomic hybridization, loss of heterozygosity, and DNA sequence analysis of single cells. Proc Natl Acad Sci U S A 96:4494–4499
Guzvic M, Braun B, Ganzer R, Burger M, Nerlich M, Winkler S, Werner-Klein M, Czyz ZT, Polzer B, Klein CA (2014) Combined genome and transcriptome analysis of single disseminated cancer cells from bone marrow of prostate cancer patients reveals unexpected transcriptomes. Cancer Res 74:7383–7394
Welty CJ, Coleman I, Coleman R, Lakely B, Xia J, Chen S, Gulati R, Larson SR, Lange PH, Montgomery B et al (2013) Single cell transcriptomic analysis of prostate cancer cells. BMC Mol Biol 14:6
Berg A, Berner A, Lilleby W, Bruland OS, Fossa SD, Nesland JM, Kvalheim G (2007) Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int J Cancer 120:1603–1609
Kollermann J, Weikert S, Schostak M, Kempkensteffen C, Kleinschmidt K, Rau T, Pantel K (2008) Prognostic significance of disseminated tumor cells in the bone marrow of prostate cancer patients treated with neoadjuvant hormone treatment. J Clin Oncol 26:4928–4933
Weckermann D, Polzer B, Ragg T, Blana A, Schlimok G, Arnholdt H, Bertz S, Harzmann R, Klein CA (2009) Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol 27:1549–1556
Cher ML, de Oliveira JG, Beaman AA, Nemeth JA, Hussain M, Wood DP Jr (1999) Cellular proliferation and prevalence of micrometastatic cells in the bone marrow of patients with clinically localized prostate cancer. Clin Cancer Res 5:2421–2425
Bianco FJ Jr, Wood DP Jr, de Gomes OJ, Nemeth JA, Beaman AA, Cher ML (2001) Proliferation of prostate cancer cells in the bone marrow predicts recurrence in patients with localized prostate cancer. Prostate 49:235–242
Mitsiades CS, Lembessis P, Sourla A, Milathianakis C, Tsintavis A, Koutsilieris M (2004) Molecular staging by RT-pCR analysis for PSA and PSMA in peripheral blood and bone marrow samples is an independent predictor of time to biochemical failure following radical prostatectomy for clinically localized prostate cancer. Clin Exp Metastasis 21:495–505
Lammers R, Giesert C, Grunebach F, Marxer A, Vogel W, Buhring HJ (2002) Monoclonal antibody 9C4 recognizes epithelial cellular adhesion molecule, a cell surface antigen expressed in early steps of erythropoiesis. Exp Hematol 30:537–545
Eisenwort G, Jurkin J, Yasmin N, Bauer T, Gesslbauer B, Strobl H (2011) Identification of TROP2 (TACSTD2), an EpCAM-like molecule, as a specific marker for TGF-beta1-dependent human epidermal Langerhans cells. J Invest Dermatol 131:2049–2057
Brownback KR, Renzulli J, Delellis R, Myers JR (2009) Small-cell prostate carcinoma: a retrospective analysis of five newly reported cases. Indian J Urol 25:259–263
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65:10946–10951
Shetye JD, Liljefors ML, Emdin SO, Frodin JE, Strigard K, Mellstedt HT, Porwit A (2004) Spectrum of cytokeratin-positive cells in the bone marrows of colorectal carcinoma patients. Anticancer Res 24:2375–2383
Deftos LJ (1991) Chromogranin A: its role in endocrine function and as an endocrine and neuroendocrine tumor marker. Endocr Rev 12:181–187
Danza G, Di SC, Rosati F, Lonetto G, Sturli N, Kacer D, Pennella A, Ventimiglia G, Barucci R, Piscazzi A et al (2012) Notch signaling modulates hypoxia-induced neuroendocrine differentiation of human prostate cancer cells. Mol Cancer Res 10:230–238
Sosa MS, Parikh F, Maia AG, Estrada Y, Bosch A, Bragado P, Ekpin E, George A, Zheng Y, Lam HM et al (2015) NR2F1 controls tumour cell dormancy via. Nat Commun 6:6170
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, Yumoto K, Berry JE, Shiozawa Y, Pienta KJ (2013) GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One 8:e61873
Kim JK, Jung Y, Wang J, Joseph J, Mishra A, Hill EE, Krebsbach PH, Pienta KJ, Shiozawa Y, Taichman RS (2013) TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia 15:1064–1074
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY et al (2013) The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 15:807–817
Lobo NA, Shimono Y, Qian D, Clarke MF (2007) The biology of cancer stem cells. Annu Rev Cell Dev Biol 23:675–699
Kleffel S, Schatton T (2013) Tumor dormancy and cancer stem cells: two sides of the same coin? Adv Exp Med Biol 734:145–179
Wang N, Docherty F, Brown HK, Reeves K, Fowles A, Lawson M, Ottewell PD, Holen I, Croucher PI, Eaton CL (2015) Mitotic quiescence, but not unique “stemness,” marks the phenotype of bone metastasis-initiating cells in prostate cancer. FASEB J 29:3141–3150
Yuen HF, Chua CW, Chan YP, Wong YC, Wang X, Chan KW (2007) Significance of TWIST and E-cadherin expression in the metastatic progression of prostatic cancer. Histopathology 50:648–658
Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, Giancotti FG (2012) The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150:764–779
Giancotti FG (2013) Mechanisms governing metastatic dormancy and reactivation. Cell 155:750–764
Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C et al (2011) Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med 208:2641–2655
Ruppender N, Larson S, Lakely B, Kollath L, Brown L, Coleman I, Coleman R, Nguyen H, Nelson PS, Corey E et al (2015) Cellular adhesion promotes prostate cancer cells escape from dormancy. PLoS One 10:e0130565
Bui AT, Laurent F, Havard M, Dautry F, Tchenio T (2015) SMAD signaling and redox imbalance cooperate to induce prostate cancer cell dormancy. Cell Cycle 14:1218–1231
Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, Schewe DM, Aguirre-Ghiso JA (2013) TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol 15:1351–1361
El Touny LH, Vieira A, Mendoza A, Khanna C, Hoenerhoff MJ, Green JE (2014) Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J Clin Invest 124:156–168
Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P (2011) Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res 71:1550–1560
Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K, Ochiya T (2014) Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal 7:ra63
Nakamura T, Shinriki S, Jono H, Guo J, Ueda M, Hayashi M, Yamashita S, Zijlstra A, Nakayama H, Hiraki A et al (2015) Intrinsic TGF-beta2-triggered SDF-1-CXCR4 signaling axis is crucial for drug resistance and a slow-cycling state in bone marrow-disseminated tumor cells. Oncotarget 6:1008–1019
Crea F, Nur Saidy NR, Collins CC, Wang Y (2015) The epigenetic/noncoding origin of tumor dormancy. Trends Mol Med 21:206–211
Lyu T, Jia N, Wang J, Yan X, Yu Y, Lu Z, Bast RC Jr, Hua K, Feng W (2013) Expression and epigenetic regulation of angiogenesis-related factors during dormancy and recurrent growth of ovarian carcinoma. Epigenetics 8:1330–1346
Gao H, Chakraborty G, Lee-Lim AP, Mavrakis KJ, Wendel HG, Giancotti FG (2014) Forward genetic screens in mice uncover mediators and suppressors of metastatic reactivation. Proc Natl Acad Sci U S A 111:16532–16537
Almog N, Ma L, Schwager C, Brinkmann BG, Beheshti A, Vajkoczy P, Folkman J, Hlatky L, Abdollahi A (2012) Consensus micro RNAs governing the switch of dormant tumors to the fast-growing angiogenic phenotype. PLoS One 7:e44001
Almog N, Briggs C, Beheshti A, Ma L, Wilkie KP, Rietman E, Hlatky L (2013) Transcriptional changes induced by the tumor dormancy-associated microRNA-190. Transcription 4:177–191
Saad F, McKiernan J, Eastham J (2006) Rationale for zoledronic acid therapy in men with hormone-sensitive prostate cancer with or without bone metastasis. Urol Oncol 24:4–12
Ottewell PD, Wang N, Meek J, Fowles CA, Croucher PI, Eaton CL, Holen I (2014) Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer 21:769–781
Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM (2015) Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis 32:335–344
Hartkopf AD, Taran FA, Wallwiener M, Hahn M, Becker S, Solomayer EF, Brucker SY, Fehm TN, Wallwiener D (2014) Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients - results from a large single-centre analysis. Eur J Cancer 50:2550–2559
Banys M, Solomayer EF, Gebauer G, Janni W, Krawczyk N, Lueck HJ, Becker S, Huober J, Kraemer B, Wackwitz B et al (2013) Influence of zoledronic acid on disseminated tumor cells in bone marrow and survival: results of a prospective clinical trial. BMC Cancer 13:480
Kangwan N, Park JM, Kim EH, Hahm KB (2014) Chemoquiescence for ideal cancer treatment and prevention: where are we now? J Cancer Prev 19:89–96
Valcourt JR, Lemons JM, Haley EM, Kojima M, Demuren OO, Coller HA (2012) Staying alive: metabolic adaptations to quiescence. Cell Cycle 11:1680–1696
Dey-Guha I, Wolfer A, Yeh AC, Albeck G, Darp R, Leon E, Wulfkuhle J, Petricoin EF III, Wittner BS, Ramaswamy S (2011) Asymmetric cancer cell division regulated by AKT. Proc Natl Acad Sci U S A 108:12845–12850
Bihani T, Chicas A, Lo CP, Lin AW (2007) Dissecting the senescence-like program in tumor cells activated by Ras signaling. J Biol Chem 282:2666–2675
Pantel K, Alix-Panabieres C (2014) Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep 3:584
Acknowledgments
The work was supported by NIH PO1 CA85859, the Pacific Northwest Prostate Cancer SPORE NIH P50 CA097186, and the Richard M. LUCAS Foundation. H.M.L. is a recipient of the Young Investigator Award from the Prostate Cancer Foundation, and a Career Development Award from the Pacific Northwest Prostate Cancer SPORE (P50 CA097186). This material is also the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington (R.L.V. is a VA Biomedical Laboratory R&D senior research career scientist and P.H.L. is a staff physician).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Morrissey, C., Vessella, R.L., Lange, P.H. et al. The biology and clinical implications of prostate cancer dormancy and metastasis. J Mol Med 94, 259–265 (2016). https://doi.org/10.1007/s00109-015-1353-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1353-4