Abstract
Purpose
Treatment of patients with laryngeal squamous cell carcinoma with radiotherapy or chemoradiation is an established alternative to laryngeal surgery in many cases, but particularly for advanced tumors without cartilage invasion. Imaging modalities face the challenge of distinguishing between posttherapeutic changes and residual disease in the complex anatomic subsite of the larynx. Guidelines concerning restaging of head and neck squamous cell carcinomas (HNSCC) are presented by the National Comprehensive Cancer Network (NCCN) and other national guidelines, but clearly defined recommendations for routine restaging particularly for laryngeal cancer are lacking.
Methods
A systematic search was carried out in PubMed to identify studies evaluating routine restaging methods after primary non-surgical treatment of laryngeal squamous cell carcinoma from 2009 to 2020.
Results
Only three studies were deemed eligible, as they included at least ≥50% patients with laryngeal squamous cell carcinoma and evaluated imaging modalities to detect residual cancer. The small number of studies in our review suggest restaging with fluoro-deoxy-glucose positron-emission tomography/computed tomography (FDG PET/CT) 3 months after initial treatment, followed by direct laryngoscopy with biopsy of the lesions identified by FDG PET/CT.
Conclusion
Studies evaluating restaging methods after organ-preserving non-surgical treatment of laryngeal carcinoma are limited. As radiotherapy (RT), chemoradiotherapy (CRT), systemic therapy followed by RT and radioimmunotherapy are established alternatives to surgical treatment, particularly in advanced laryngeal cancers, further studies are needed to assess and compare different imaging modalities (e.g. PET/CT, MRI, CT, ultrasound) and clinical diagnostic tools (e.g., video laryngoscopy, direct laryngoscopy) to offer patients safe and efficient restaging strategies. PET or PET/CT 3 months after initial treatment followed by direct laryngoscopy with biopsy of the identified lesions has the potential to reduce the number of unnecessary laryngoscopies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laryngeal carcinoma occurs in 3/100,000 persons [1]. Early laryngeal cancers are usually treated by surgery, with favorable results [2, 3]. Currently, patients with advanced laryngeal squamous cell carcinoma (T3, T4) are offered radical surgery (total or near-total laryngectomy) followed by adjuvant therapy or systemic chemoradiation (induction chemotherapy in selected cases) as treatment options [4,5,6,7,8,9]. Factors like cartilage invasion, voice, swallowing, quality of life, and the patient’s preferences contribute to choosing the appropriate treatment modality [10]. Unfortunately, locally advanced (T3, T4) laryngeal cell carcinomas still show 5‑year overall survival rates of less than 50% [11]. Furthermore, in T2–T4 laryngeal carcinoma, a local or locoregional recurrence rate between 25 and 50% after radiotherapy (RT) or chemoradiotherapy (CRT) is detectable [12]. These numbers emphasize the importance of a structured follow-up for this patient group to detect failure of treatment as early as possible. Planned restaging for tumor response evaluation should be one of the first steps in a patient’s follow-up. Clinical symptoms of residual cancer like pain, dysphagia, hoarseness, and respiratory distress can also occur secondary to radiation toxicity, which complicates diagnosis. Imaging modalities like computed tomography (CT) or magnetic resonance imaging (MRI) sometimes have difficulty in differentiating between posttherapeutic changes (like edema and protracted mucositis/laryngitis) and residual disease, notably in the complex anatomic subsite of the larynx. In general, repeating pretreatment baseline imaging of the primary within 6 months of treatment is recommended for head and neck squamous cell carcinoma (HNSCC). In 2016, de Bree et al. showed that positron-emission tomography/computed tomography (PET/CT) in case of suspected recurrence after (chemo)radiotherapy of laryngeal cancer reduced the number of unnecessary laryngoscopies [13]. However, although guidelines concerning routine restaging of HNSCC are presented by the National Comprehensive Cancer Network (NCCN) and national guidelines, clearly defined recommendations for restaging particularly for laryngeal cancer are missing [14, 15]. Therefore, the objective of this review is to evaluate methods for routine restaging and evaluation of tumor response (partial or complete remission) for laryngeal cancer after treatment with radio- or chemoradiotherapy. We discuss current literature on restaging methods after primary RT, CRT, or radioimmunotherapy of laryngeal squamous cell carcinoma.
Methods
A systematic literature search of original research articles published in English within the last 10 years (until July 2020) was conducted in PubMed using the search term (laryngeal cancer OR laryngeal carcinoma OR larynx cancer OR larynx carcinoma) AND (radiation therapy OR chemoradiotherapy OR chemoradiation OR radioimmunotherapy) AND (PET OR PET/CT OR PET/MRI OR CT OR MRI OR sonography OR ultrasound OR laryngoscopy OR microlaryngoscopy). The resulting list of articles was screened for duplicates by a public reference manager (Mendeley 1.19.4, Mendeley Ltd, London, UK). Titles and abstracts were screened by three reviewers with regard to the PICO (Patients, Intervention, Comparison, Outcome) framework. Included were only studies with at least ≥50% patients with laryngeal squamous cell carcinoma after primary RT, CRT, or radioimmunotherapy. Only studies evaluating routine restaging strategies were included, studies evaluating diagnostic work-up of suspected recurrence or follow-up studies were excluded. The outcome of interest was detection of residual cancer. Inclusion and exclusion criteria are summarized in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Fig. 1).
Results
Literature search
After applying the filters “<10 years,” “English language,” and “human species,” the PubMed literature search showed 437 references. None had to be excluded due to duplicates. After screening of titles and abstracts, the analysis of 28 full-text articles identified three studies (Table 1) which met the inclusion criteria of the review. 409 studies were excluded because they were case reports, reviews, clinical guidelines, tumor entities other than squamous cell carcinoma of the larynx, received surgical treatments, and/or did not evaluate restaging techniques. On screening full-text articles, eight studies were excluded as they included less than 50% of patients with laryngeal cancer in their cohort. 15 studies were excluded because they did not evaluate the detection of residual cancer after primary RT, CRT, or radioimmunotherapy. Two studies appeared to be reviews of literature and not original research articles when screening the full-text articles. Data analysis was performed to compare restaging methods after organ-preserving treatment of laryngeal carcinoma with RT, CRT, or radioimmunotherapy regarding their diagnostic value. Therefore, the following information was extracted from studies analyzed: name of first author, publication date, type of study, patient number, tumor stage, tumor localization, treatment information, time to imaging after therapy, posttherapeutic imaging modalities, sensitivity, specificity, accuracy, positive predictive values, negative predictive values, and median follow-up time (Table 1). In all of the studies listed in Table 1, confirmation of residual tumor was obtained by imaging follow-up and/or histological examination.
Findings from clinical studies
Table 1 summarizes three articles with regard to restaging methods after organ-preserving treatment of laryngeal carcinoma with RT or CRT. In the above-mentioned studies, none of the patients were treated with radioimmunotherapy. Overall restaging modalities were evaluated in 89 patients, of whom 53 (59.6%) had advanced laryngeal cancer (tumor stage T3, T3–T4, T4). 59 (66.3%) patients were treated with RT, 30 (33.7%) with CRT. One study obtained data retrospectively with 28 patients included in the study, whereas two studies were prospective with 47 and 14 evaluated patients. The time to imaging after RT or CRT varied from 2 to 12 months. The median time for which the patients were followed up was 24 to 35 months.
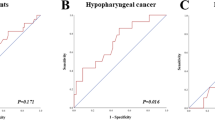
The restaging methods discussed in those studies comprise [18F]-2-fluoro-2-deoxy-D-glucose ([18F] FDG) positron-emission tomography (FDG PET), [18F]-fluoro-30-deoxy-L-thymidine ([18F] FLT) positron-emission tomography (FLT PET), and C‑11 Methionine (MET) PET. FDG is the most widely used radioactive PET tracer in oncology. Drawbacks of FDG are the physiological uptake in muscles of the larynx and the uptake by inflammatory or reactive tissues occurring after radiotherapy. The sensitivity of FDG PET to detect residual tumor after RT or CRT was 33% in one study [17], and 67 and 75% in two other studies [16, 18]. Been et al. and Mayo et al. reported a specificity of 86 and 85% for FDG PET, respectively [16, 17], whereas Wedman et al. showed a lower specificity of 53% [18]. The PPV in the study by Mayo et al. for FDG PET was 40% [17], which was lower compared to the PPV of 67 and 62% detected in the studies of Been et al. and Wedman et al., respectively [16, 18]. All three studies showed a comparable NPV, with 86, 81, and 73%, respectively [16,17,18]. All studies investigated imaging 2–12 months after finishing radiotherapy. In the study of Wedman et al., 17 patients were included between 1 and 5 years after finishing therapy. Therefore, imaging was not performed with regard to restaging and the results of these patients are not included in this review. Been et al. included FLT PET as a restaging modality in their study and found a sensitivity of 33% and a specificity of 100%. FLT is phosphorylated by thymidine kinase 1 and trapped in the cell. Its activity is increased in proliferating cells like malignant cancer cells. After radiotherapy, the sensitivity of FDG to detect residual tumor was higher as compared to FLT [16]. Wedman et al. also described MET PET as a restaging method, which had a sensitivity of 53% and a specificity of 76%. C‑11 MET is an established radiopharmaceutical and has been successfully used for visualizing primary head and neck cancer. The uptake of amino acids is high in tumor cells but low in inflammatory tissues and could therefore be a good alternative to distinguish residual tumor cells from posttherapeutic inflammation after radiotherapy. The PPV of MET was not significantly higher than the PPV obtained with FDG. Therefore, this study implies that MET PET cannot be used to select patients for a direct laryngoscopy compared to FDG PET.
Discussion
Currently, the American National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology for head and neck cancers (version 3.2019–September 16, 2019) [19] recommend a clinical assessment 4–8 weeks after treatment of laryngeal carcinoma with CRT or RT alone. If the clinical assessment shows tumor response, a CT of the primary cancer site and the neck and/or an MRI with contrast should be performed within 8–12 weeks or an FDG PET/CT should be carried out within 12 weeks to assess the extent of the disease. In case the clinical assessment suggests residual primary tumor, persistent disease, or disease progression, a CT and/or an MRI with contrast within 4–8 weeks or an FDG PET/CT should be considered. Accurate diagnostic tools are important to ensure that the correct treatment recommendations are made [20]. Therefore, sensitivity, specificity, accuracy, PPV, and NPV are important parameters to determine the optimal restaging modality for patients with laryngeal squamous cell carcinoma who have been treated by RT or CRT.
The current 2A recommendations from the NCCN are based on a review by Kutler et al. [21] evaluating the role of neck dissection following definitive CRT [21]. Restaging methods particularly for laryngeal cancer treated with induction chemotherapy followed by RT, RT, alone or CRT are lacking. Despite this, in recent decades, refinements in radiotherapy technique and protocols for induction chemotherapy have made non-surgical organ-preserving treatment methods for laryngeal cancer an established alternative to laryngectomy [7, 22].
Focusing the literature search on restaging methods after RT, CRT, or radioimmunotherapy of laryngeal squamous cell carcinoma limited the evaluated restaging methods to FDG PET/CT (Table 1). Other publications evaluated in the full-text screening analyzed restaging methods after RT, CRT, or radioimmunotherapy for patients with HNSCC, including other tumor locations. Those studies examined computerized tomography (CT), magnetic resonance imaging (MRI), diffusion-weighted MRI (DW MRI), outpatient video laryngoscopy with white light and narrow-band imaging (NBI), and direct laryngoscopy with white light and NBI, in addition to FDG PET/CT and direct laryngoscopy. These studies were excluded as they included less than 50% of patients diagnosed with laryngeal cancer or evaluated imaging modalities that detect recurrent tumor lesions rather than restaging methods.
In a heterogenous HNSCC patient cohort, sensitivity for detecting recurrent or residual tumor after RT or CRT was determined for direct laryngoscopy under general anesthesia with white light and NBI (100%) in 68 patients, compared to 88% in outpatient video laryngoscopy with white light and NBI in 66 patients [23]. Next, DW MRI showed a sensitivity of 94% in a study with 46 patients [24] and a sensitivity of 69% in an article evaluating 70 patients [25]. Another study investigating the use of dynamic contrast-enhanced MRI in predicting early response to CRT in HNSCC patients found a sensitivity and specificity of 89.3 and 73.5%, respectively [26]. The sensitivity for MRI was 72% in a study analyzing 46 patients [24], whereas CT showed a sensitivity of 68% in a study with 111 patients [27]. The most specific restaging modalities were outpatient video laryngoscopy with white light and NBI, as well as direct laryngoscopy with white light and NBI (both 92%) [23]. The specificity of DW MRI varied between 100% [24] and 77% [25], whereas CT demonstrated a higher specificity (88%) [27] than MRI (57%) [24]. The PPV for DW MRI was either 100% [24] or 75% [25], followed by 79% for outpatient video laryngoscopy with white light and NBI and direct laryngoscopy with white light and NBI [23]. The lowest PPV were evaluated for MRI (52%) [24] and CT (50%) [27]. NPV were especially high in outpatient video laryngoscopy with white light and NBI and direct laryngoscopy with white light and NBI (96 and 100%) [23]. CT (8/93%) [27] showed similar NPV to DW MRI (71% [25] and 97% [24]), followed by MRI with 76% [24].
Other authors have investigated the use of FDG PET/CT in restaging after HNSCC [25, 27,28,29,30,31,32]. The patient cohorts were not focused on laryngeal carcinoma. To our knowledge, the three articles analyzed in this review are the only publications focusing on restaging of laryngeal carcinoma after RT, CRT, or radioimmunotherapy in the past 10 years. For the detection of residual or recurrent HNSCC, the sensitivity of FDG PET/CT ranges from 71 to 97%, whereas specificity ranges from 46 to 92%, the PPV ranges from 64 to 71%, and the NPV from 86 to 98% [25, 27, 31, 33]. Analyzing the studies in Table 1, the sensitivity of FDG PET/CT for detecting residual laryngeal carcinoma ranges from 33 to 75%, specificity from 53to 86%, PPV from 40 to 67%, and NPV from 73 to 86%.
Literature on the value of FDG PET/CT imaging in the diagnosis and staging of patients with laryngeal carcinoma shows a higher sensitivity (100%) than MRI/CT (93.3%); additionally, FDG PET/CT is able to detect regional nodal and distant metastasis, as well as synchronous tumors [34]. Reasons for the lower accuracy of FDG PET/CT in restaging of laryngeal carcinoma after organ-preserving non-surgical treatment may be false-positive results due to inflammation, edema, or protracted mucositis/laryngitis, which increase the necessity of laryngoscopies with biopsies under general anesthesia. It is important to note that several studies have demonstrated that FDG PET/CT is less sensitive early after treatment and is best carried out 12 weeks post CRT to minimize the risk of false-positive results [19, 35,36,37]. In our review, the time between therapy and imaging with FDG PET/CT was 2–12 months. Other issues with FDG PET/CT are the high costs and availability of the procedure. An analysis of Smith et al. published in 2016 indicates that PET/CT-guided patient management is cost effective in the long-term in a UK-based patient cohort [38]. Another possible restaging method is the use of FLT or MET instead of FDG in positron-emission tomography. These methods showed lower sensitivities and NPV, but higher values for specificity and PPV. The overall tracer uptake was significantly lower as compared to FDG, and tumor-to-background ratios were comparable or lower than the ratio obtained with FDG [16, 18]. Still, the authors deemed both modalities feasible for visualizing laryngeal cancer.
Positive results in restaging with FDG PET/CT need to be confirmed by biopsy during a laryngoscopy under general anesthesia. In the study of de Bree et al., laryngoscopy without previous imaging showed a PPV of only 32% [13] due to a high number of false-positive results, which in this study equaled an unnecessary indication for a laryngoscopy. Zabrodsky et al. found a PPV of 79% for direct laryngoscopy when combined with narrow-band imaging [23]. Outpatient video laryngoscopy with narrow-band imaging showed the same PPV (79%) and specificity (92%) as direct laryngoscopy, but the sensitivity was higher in direct laryngoscopy (100%) compared to video laryngoscopy (88%) [23] and equal to a study by Terhaard et al. published in 2001 [39].
When looking into restaging laryngeal cancer with CT or MRI after treatment with systemic therapy and/or radiotherapy, our literature research did not reveal any studies published within the past 10 years. With the mentioned low PPV for MRI (52%) [24] and CT (50%) [27], both modalities cannot reliably differentiate residual or recurrent disease from postirradiation changes in laryngeal carcinoma, which has been demonstrated previously by other reviews [40].
Ultrasound, another important diagnostic tool [41], was also not evaluated by any study in the past 10 years looking at restaging of laryngeal cancer treated with RT or CRT. A prospective study by de Fiori et al. published in 2016 demonstrated a sensitivity of 85.7% and a specificity of 100% for ultrasound-guided transcutaneous biopsy in 19 patients with laryngeal and hypopharyngeal carcinoma [42]. Contrast-enhanced ultrasound already plays a role in therapy control and monitoring of liver tumors and could at least be helpful to support treatment response evaluation of cervical lymph nodes [43, 44]. Endoscopic ultrasound offers high-resolution imaging of endolaryngeal structures and their pathological changes [45]. Further studies are needed to evaluate the role of ultrasound in restaging after organ-preserving non-surgical treatment of laryngeal carcinoma.
Overall, FDG PET/CT in combination with direct laryngoscopy seems to be the most favorable restaging method to assess laryngeal squamous cell carcinoma after treatment with RT or CRT. With Mehanna et al. demonstrating that FDG PET/CT 12 weeks after primary non-surgical treatment can reduce the necessity of salvage neck dissection and de Bree et al. showing that FDG PET/CT in restaging lowers the need for direct laryngoscopies under general anesthesia, insurance policies are willing to cover the costs of the procedure [13, 46, 47]. To avoid false-positive results, but still remain eligible for surgical treatment, FDG PET/CT should be performed 12 weeks after the end of primary non-surgical treatment [48]. Finally, there is a need for many more prospective studies evaluating the restaging modalities, especially FDG PET/CT and DW MRI, for laryngeal squamous cell carcinoma after systemic therapy with radiotherapy, CRT, or RT alone [49].
Conclusion
Studies evaluating restaging methods after organ-preserving non-surgical treatment of laryngeal carcinoma are limited. As RT, CRT, and systemic therapy followed by RT are established alternatives for surgical treatment particularly in advanced laryngeal cancers without cartilage invasion, further prospective studies are needed to assess and compare different imaging modalities (e.g., FDG PET/CT, MRI, CT, ultrasound) and clinical diagnostic tools (e.g., video laryngoscopy, direct laryngoscopy with NBI) to offer patients safe and efficient restaging strategies. The small number of studies in our review does not allow for clear suggestions regarding restaging of laryngeal cancer. However, looking at the available data and guidelines, FDG PET/CT 3 months after initial treatment followed by direct laryngoscopy with biopsy of the lesions identified by FDG PET/CT is a reasonable approach for T2–T4 laryngeal carcinomas (Fig. 2). This procedure has the potential to reduce the number of unnecessary laryngoscopies. T1 glottic laryngeal cancers usually allow solely clinical evaluation due to their location and size. After a negative FDG PET/CT or unremarkable laryngoscopy with biopsy, regular clinical evaluation with annual repetition of the pretherapeutic imaging is a reasonable follow-up strategy. The development of clinical guidelines specifically for restaging of laryngeal carcinoma after non-surgical treatment is necessary.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics. CA Cancer J Clin 69:7–34
Dey P, Arnold D, Wight R, MacKenzie K, Kelly C, Wilson J (2002) Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev 2:CD2027
Warner L, Chudasama J, Kelly CG, Loughran S, McKenzie K, Wight R, Dey P (2014) Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev 12:CD2027
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098
Forastiere AA, Ismaila N, Lewin JS, Nathan CA, Adelstein DJ, Eisbruch A, Fass G, Fisher SG, Laurie SA, Le Q‑T et al (2018) Use of larynx-preservation strategies in the treatment of laryngeal cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol 36:1143–1169
Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA et al (2013) Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 31:845–852
Dietz A, Wichmann G, Kuhnt T, Pfreundner L, Hagen R, Scheich M, Kölbl O, Hautmann MG, Strutz J, Schreiber F et al (2018) Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy—final results of the larynx organ preservation trial DeLOS-II. Ann Oncol 29:2105–2114
Haussmann J, Tamaskovics B, Bölke E, Djiepmo-Njanang F‑J, Kammers K, Corradini S, Hautmann M, Ghadjar P, Maas K, Schuler PJ et al (2019) Addition of chemotherapy to hyperfractionated radiotherapy in advanced head and neck cancer—a meta-analysis. Strahlenther Onkol 195:1041–1049
Hoebers F, Rios E, Troost E, van den Ende P, Kross K, Lacko M, Lalisang R, Kremer B, de Jong J (2013) Definitive radiation therapy for treatment of laryngeal carcinoma. Strahlenther Onkol 189:834–841
Scherl C, Mantsopoulos K, Semrau S, Fietkau R, Kapsreiter M, Koch M, Traxdorf M, Grundtner P, Iro H (2017) Management of advanced hypopharyngeal and laryngeal cancer with and without cartilage invasion. Auris Nasus Larynx 44:333–339
Megwalu UC, Sikora AG (2014) Survival outcomes in advanced laryngeal cancer. JAMA Otolaryngol Head Neck Surg 140:855–860
Zbären P, de Bree R, Takes RP, Rinaldo A, Ferlito A (2013) Which is the most reliable diagnostic modality for detecting locally residual or recurrent laryngeal squamous cell carcinoma after (chemo)radiotherapy? Eur Arch Otorhinolaryngol 270:2787–2791
de Bree R, van der Putten L, van Tinteren H, Wedman J, Oyen WJG, Janssen LM, van den Brekel MWM, Comans EFI, Pruim J, Takes RP et al (2016) Effectiveness of an 18F-FDG-PET based strategy to optimize the diagnostic trajectory of suspected recurrent laryngeal carcinoma after radiotherapy: The RELAPS multicenter randomized trial. Radiother Oncol 118:251–256
Fenton M, Foote RL, Galloway T, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, Jimeno A, Leizman D, Pinto HA et al (2020) Continue NCCN guidelines panel disclosures NCCN gratefully acknowledges the following subcommittee member for her contributions on the development of the principles of imaging (IMG-A) NCCN guidelines version 2.2020 head and neck cancers
Leitlinienprogramm Onkologie (2019) S3-Leitlinie Diagnostik, Therapie und Nachsorge des Larynxkarzinoms
Been LB, Hoekstra HJ, Suurmeijer AJH, Jager PL, van der Laan BFAM, Elsinga PH (2009) 18F]FLT-PET and [18F]FDG-PET in the evaluation of radiotherapy for laryngeal cancer. Oral Oncol 45:e211–e215
Mayo Z, Seyedin SN, Mallak N, Mott SL, Menda Y, Graham M, Anderson C (2019) Clinical utility of pretreatment and 3‑month 18F-fluorodeoxyglucose positron emission tomography/computed tomography standardized uptake value in predicting and assessing recurrence in T3–T4 laryngeal carcinoma treated with definitive radiation. Ann Otol Rhinol Laryngol 128:595–600
Wedman J, Pruim J, van der Putten L, Hoekstra OS, de Bree R, van Dijk BAC, van der Laan BFAM (2019) Is C‑11 methionine PET an alternative to 18‑F FDG-PET for identifying recurrent laryngeal cancer after radiotherapy? Clin Otolaryngol 44:124–130
Foote RL, Gillison ML, Haddad RI, Hicks WL, Hitchcock YJ, Jimeno A, Leizman D, Maghami E, Pinto HA, Ridge JA et al (2019) Continue NCCN guidelines panel disclosures
Cozzi L, Franzese C, Fogliata A, Franceschini D, Navarria P, Tomatis S, Scorsetti M (2019) Predicting survival and local control after radiochemotherapy in locally advanced head and neck cancer by means of computed tomography based radiomics. Strahlenther Onkol 195:805–818
Kutler DI, Patel SG, Shah JP (2004) The role of neck dissection following definitive chemoradiation. Oncology 18:993–998 (discussion 999, 1003–4, 1007)
Dietz A, Wiegand S, Kuhnt T, Wichmann G (2019) Laryngeal preservation approaches: considerations for new selection criteria based on the deLOS-II trial. Front Oncol 9:625
Zabrodsky M, Lukes P, Lukesova E, Boucek J, Plzak J (2014) The role of narrow band imaging in the detection of recurrent laryngeal and hypopharyngeal cancer after curative radiotherapy. Biomed Res Int 2014:1–9
Tshering Vogel DW, Zbaeren P, Geretschlaeger A, Vermathen P, De Keyzer F, Thoeny HC (2013) Diffusion-weighted MR imaging including bi-exponential fitting for the detection of recurrent or residual tumour after (chemo)radiotherapy for laryngeal and hypopharyngeal cancers. Eur Radiol 23:562–569
Driessen JP, Peltenburg B, Philippens MEP, Huijbregts JE, Pameijer FA, de Bree R, Janssen LM, Terhaard CHJ (2019) Prospective comparative study of MRI including diffusion-weighted images versus FDG PET-CT for the detection of recurrent head and neck squamous cell carcinomas after (chemo)radiotherapy. Eur J Radiol 111:62–67
Guo W, Luo D, Chen X, Lin M, Li L, Zhao Y, Yang L, Hu L, Zhao X, Zhou C (2017) Dynamic contrast-enhanced magnetic resonance imaging for pretreatment prediction of early chemo-radiotherapy response in larynx and hypopharynx carcinoma. Oncotarget 8:33836–33843
Bae JS, Roh J‑L, Lee S, Kim S‑B, Kim JS, Lee JH, Choi S‑H, Nam SY, Kim SY (2012) Laryngeal edema after radiotherapy in patients with squamous cell carcinomas of the larynx and hypopharynx. Oral Oncol 48:853–858
Mori M, Yabuki K, Taguchi T, Nishimura G, Takahashi M, Komatsu M, Oridate N (2015) Long-term results of survival analysis after a 5-year follow-up: efficacy of fluoro-2-deoxy-D-glucose positron emission tomography to evaluate responses to concurrent chemoradiotherapy for head and neck squamous cell carcinoma. Auris Nasus Larynx 42:263
Sagardoy T, Fernandez P, Ghafouri A, Digue L, Haaser T, de Clermont-Galleran H, Castetbon V, de Monès E (2016) Accuracy of (18) FDG PET-CT for treatment evaluation 3 months after completion of chemoradiotherapy for head and neck squamous cell carcinoma: 2‑year minimum follow-up. Head Neck 38(1):E1271–6
Mori M, Tsukuda M, Horiuchi C, Matsuda H, Taguchi T, Takahashi M, Nishimura G, Komatsu M, Niho T, Sakuma N et al (2011) Efficacy of fluoro-2-deoxy-D-glucose positron emission tomography to evaluate responses to concurrent chemoradiotherapy for head and neck squamous cell carcinoma. Auris Nasus Larynx 38:724–729
Uzel EK, Ekmekcioglu O, Elicin O, Halac M, Uzel OE (2013) Is FDG-PET-CT a valuable tool in prediction of persistent disease in head and neck cancer. Asian Pac J Cancer Prev 14:4847–4851
Nelissen C, Sherriff J, Jones T, Guest P, Colley S, Sanghera P, Hartley A (2017) The role of positron emission tomography/computed tomography imaging in head and neck cancer after radical chemoradiotherapy: a single institution experience. Clin Oncol 29:753–759
Nelissen C, Sherriff J, Jones T, Guest P, Colley S, Sanghera P, Hartley A (2017) The role of positron emission tomography/computed tomography imaging in head and neck cancer after radical chemoradiotherapy: a single institution experience. Clin Oncol (R Coll Radiol) 29:753–759
Tatar G, Cermik TF, Karagoz Y, Gundogan C, Karacetin D, Yildiz E, Yigit O (2018) The value of whole-body contrast-enhanced 18F-FDG PET/CT imaging in the diagnosis and staging of patients with laryngeal carcinoma. Nucl Med Commun 39:334–342
Isles MG, McConkey C, Mehanna HM (2008) A systematic review and meta-analysis of the role of positron emission tomography in the follow up of head and neck squamous cell carcinoma following radiotherapy or chemoradiotherapy. Clin Otolaryngol 33:210–222
Mehanna H, McConkey CC, Rahman JK, Wong W‑L, Smith AF, Nutting C, Hartley AG, Hall P, Hulme C, Patel DK et al (2017) PET-NECK: a multicentre randomised phase III non-inferiority trial comparing a positron emission tomography–computerised tomography-guided watch-and-wait policy with planned neck dissection in the management of locally advanced (N2/N3) nodal metastases in patients with squamous cell head and neck cancer. Health Technol Assess 21:1–122
Gupta T, Master Z, Kannan S, Agarwal JP, Ghsoh-Laskar S, Rangarajan V, Murthy V, Budrukkar A (2011) Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 38:2083–2095
Smith AF, Hall PS, Hulme CT, Dunn JA, McConkey CC, Rahman JK, McCabe C, Mehanna H (2017) Cost-effectiveness analysis of PET-CT-guided management for locally advanced head and neck cancer. Eur J Cancer 85:6–14
Terhaard CH, Bongers V, van Rijk PP, Hordijk GJ (2001) F‑18-fluoro-deoxy-glucose positron-emission tomography scanning in detection of local recurrence after radiotherapy for laryngeal/pharyngeal cancer. Head Neck 23:933–941
Brouwer J, Hooft L, Hoekstra OS, Riphagen II, Castelijns JA, de Bree R, Leemans CR (2008) Systematic review: accuracy of imaging tests in the diagnosis of recurrent laryngeal carcinoma after radiotherapy. Head Neck 30:889–897
Künzel J, Strieth S, Wirth G, Bozzato A (2018) Ultrasound in the re-staging of cervical metastases after chemoradiotherapy for head and neck cancer. Ultraschall Med 39:659–666
De Fiori E, Conte G, Ansarin M, De Benedetto L, Bonello L, Alterio D, Maffini F, Bellomi M, Preda L (2016) The role of ultrasound-guided transcutaneous tru-cut biopsy in diagnosing untreated and recurrent laryngo-hypopharyngeal masses. Eur J Radiol 85:158–163
Wendl CM, Müller S, Meier J, Fellner C, Eiglsperger J, Gosau M, Prantl L, Stroszczynski C, Jung EM (2012) High resolution contrast-enhanced ultrasound and 3‑tesla dynamic contrast-enhanced magnetic resonance imaging for the preoperative characterization of cervical lymph nodes: first results. Clin Hemorheol Microcirc 52:153–166
Rennert J, Wiesinger I, Beyer LP, Schicho A, Stroszczynski C, Wiggermann P, Jung EM (2019) Color coded perfusion analysis and microcirculation imaging with contrast enhanced ultrasound (CEUS) for post-interventional success control following thermal ablative techniques of primary and secondary liver malignancies. Clin Hemorheol Microcirc 73:73–83
Arens C, Kraft M (2016) Endoscopic ultrasound of the larynx. Curr Opin Otolaryngol Head Neck Surg 24:128–134
Mehanna H, Wong W‑L, McConkey CC, Rahman JK, Robinson M, Hartley AGJ, Nutting C, Powell N, Al-Booz H, Robinson M et al (2016) PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 374:1444–1454
Hermann RM, Christiansen H (2016) Restaging PET-CT after radiochemotherapy can spare patients with advanced head and neck cancer from neck dissection provided they are in complete remission. Strahlenther Onkol 192:589–591
De Felice F, Musio D, Tombolini V (2015) Follow-up in head and neck cancer: a management dilemma. Adv Otolaryngol. https://doi.org/10.1155/2015/703450
Suárez-Quintanilla J, Fernández Cabrera A, Sharma S (2020) Anatomy, head and neck, larynx. StatPearls Publishing, Treasure Island (FL)
Funding
This research received no external funding.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
C.S., B.H., and J.K. designed the review. C.S., B.H., and J.K. performed the literature search. C.S., C.B., MG.H., and J.K. analyzed the obtained articles. C.S., I.U., J.M., and J.K. wrote the paper. C.S. and J.K. collected and assembled the data. J.G., J.R., EM.J., MG.H., C.B., and H.M. revised and edited the manuscript critically. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
C. Seebauer, B. Hackenberg, J. Grosse, J. Rennert, E.-M. Jung, I. Ugele, I. Michaelides, H. Mehanna, M.G. Hautmann, C. Bohr, and J. Künzel declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seebauer, C.T., Hackenberg, B., Grosse, J. et al. Routine restaging after primary non-surgical treatment of laryngeal squamous cell carcinoma—a review. Strahlenther Onkol 197, 167–176 (2021). https://doi.org/10.1007/s00066-020-01706-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01706-9