Abstract
Purpose
Radiotherapy (RT) for peripheral organs can affect circulating lymphocytes and cause lymphopenia. We aimed to investigate RT-related lymphopenia in patients with hepatocellular carcinoma (HCC).
Methods
Medical records of 920 patients who received RT for HCC during 2001–2016 were reviewed. Total lymphocyte count (TLC) were obtained and analyzed for clinical outcome. Acute severe lymphopenia (ASL) was defined as TLC <500/μL within the first 3 months of the start of RT.
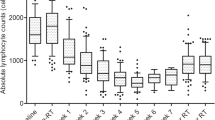
Results
The median TLCs before and 1 month after the start of RT were 1120 and 310/μl, respectively, and the TLCs did not recover to their initial level after 1 year. Overall, 87.4% of patients developed ASL. The median overall survival was 13.6 and 46.7 months for patients with and without ASL, respectively (p < 0.001). ASL was independently associated with poor overall survival with a hazard ratio (HR) of 1.40; 95% confidence interval (CI), 1.02–1.91 (p = 0.035). In the multivariate analysis, larger planning target volume (HR, 1.02; 95% CI, 1.01–1.03; p < 0.001) and lower baseline TLC (HR, 0.86; 95% CI, 0.82–0.91; p < 0.001) were significantly associated with an increased risk of ASL, while hypofractionation (stereotactic body RT: HR, 0.19; 95% CI, 0.07–0.49; p = 0.001) was significantly associated with a reduced risk of ASL.
Conclusion
Acute severe lymphopenia after RT was associated with poor overall survival in patients with HCC. Stereotactic body RT may reduce the risk of ASL. Further attention to and research on the cause, prevention, and reversal of this phenomenon are needed.
Zusammenfassung
Zielsetzung
Strahlentherapie (RT) für Peripherieorgane kann zirkulierende Lymphozyten schädigen und Lymphopenie verursachen. Wir haben zum Ziel, RT-bezogene Lymphopenie bei Patienten mit Leberkarzinom zu erforschen (HCC).
Methoden
Überprüft wurden medizinische Aufzeichnungen von 920 Patienten, die in den Jahren 2001–2016 eine Strahlentherapie bei HCC erhalten hatten. Die Gesamtanzahl der Lymphozyten (TLC) wurde bestimmt und für klinische Ergebnisse analysiert. Akute schwere Lymphopenie (ASL) wurde als TLC <500/μl innerhalb der ersten 3 Monate nach Beginn der RT definiert.
Ergebnisse
Der Mittelwert der TLC vor und 1 Monat nach Beginn der RT lag bei jeweils 1120 und 310/μl, und die TLC erreichte auch nach 1 Jahr nicht ihr Ursprungsniveau. Insgesamt entwickelten 87,4% der Patienten eine ASL. Die mittlere Gesamtüberlebensrate lag jeweils bei 13,6 und 46,7 Monaten bei Patienten mit und ohne ASL (p < 0,001). ASL wurde unabhängig mit schlechter Überlebensrate in Verbindung gesetzt (Hazard Ratio [HR] 1,40; 95%-Konfidenzintervall [KI] 1,02–1,91; p = 0,035). In der mehrdimensionalen Analyse wurden geplante Zielmenge (HR 1,02; 95%-KI 1,01–1,03; p < 0,001) und geringere TLC-Basislinie (HR 0,86; 95%-KI 0,82–0,91; p < 0,001) maßgeblich mit einem erhöhten Risiko von ASL in Verbindung gesetzt, während Hypofraktionierung (stereotaktische Körper-RT: HR 0,19; 95%-KI 0,07–0,49; p = 0,001) mit einem reduzierten Risiko von ASL in Verbindung gesetzt wird.
Schlussfolgerung
ASL nach RT wurde mit schlechter allgemeiner Überlebensrate bei Patienten mit HCC in Verbindung gebracht. Stereotaktische Körper-RT kann das Risiko von ASL reduzieren. Zusätzliche Aufmerksamkeit und Ursachenforschung, Verhütung und Umkehr dieses Phänomens werden benötigt.



Similar content being viewed by others
References
Boon T, Coulie PG, VandenEynde B (1997) Tumor antigens recognized by T cells. Immunol Today 18(6):267–268
Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, Chapman PB, Schwartz GK, Allison JP, Wolchok JD (2010) Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 116(7):1767–1775
Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, Brock M, Balmanoukian A, Ye X (2015) Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J Natl Compr Canc Netw 13(10):1225–1231
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S (2011) Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res 17(16):5473–5480
Huang J, DeWees TA, Badiyan SN, Speirs CK, Mullen DF, Fergus S, Tran DD, Linette G, Campian JL, Chicoine MR, Kim AH, Dunn G, Simpson JR, Robinson CG (2015) Clinical and dosimetric predictors of acute severe Lymphopenia during radiation therapy and concurrent Temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys 92(5):1000–1007
Liu LT, Chen QY, Tang LQ, Guo SS, Guo L, Mo HY, Chen MY, Zhao C, Guo X, Qian CN, Zeng MS, Bei JX, Tan J, Chen S, Hong MH, Shao JY, Sun Y, Ma J, Mai HQ (2018) The prognostic value of treatment-related Lymphopenia in Nasopharyngeal carcinoma patients. Cancer Res Treat 50(1):19–29
Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, Korff T, von Deimling A, Unterberg A, Beckhove P, Herold-Mende C (2011) Effector T‑cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin. Cancer Res 17(13):4296–4308
Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW (2011) The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 105(1):93–103
Campian JL, Sarai G, Ye X, Marur S, Grossman SA (2014) Association between severe treatment-related lymphopenia and progression-free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck 36(12):1747–1753
Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, Rosati LM, Hacker-Prietz A, Assadi RK, Saeed AM, Pawlik TM, Jaffee EM, Laheru DA, Tran PT, Weiss MJ, Wolfgang CL, Ford E, Grossman SA, Ye X, Ellsworth SG (2016) Lymphocyte-sparing effect of Stereotactic body radiation therapy in patients with Unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 94(3):571–579
Wild AT, Ye X, Ellsworth SG, Smith JA, Narang AK, Garg T, Campian J, Laheru DA, Zheng L, Wolfgang CL, Tran PT, Grossman SA, Herman JM (2015) The association between Chemoradiation-related Lymphopenia and clinical outcomes in patients with locally advanced pancreatic Adenocarcinoma. Am J Clin Oncol 38(3):259–265
Button LN, Dewolf WC, Newburger PE, Jacobson MS, Kevy SV (1981) The effects of irradiation on blood components. Transfusion 21(4):419–426
Nakamura N, Kusunoki Y, Akiyama M (1990) Radiosensitivity of CD4 or CD8 positive human T‑lymphocytes by an in vitro colony formation assay. Radiat Res 123(2):224–227
Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E (2013) The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 31(2):140–144
Petrini B, Wasserman J, Blomgren H, Baral E, Strender LE, Wallgren A (1979) Blood lymphocyte subpopulations in breast cancer patients following post-operative adjuvant chemotherapy or radiotherapy. Clin Exp Immunol 38(2):361–365
Rudra S, Hui C, Rao YJ, Samson P, Lin AJ, Chang X, Tsien C, Fergus S, Mullen D, Yang D, Thotala D, Hallahan D, Campian JL, Huang J (2018) Effect of radiation treatment volume reduction on Lymphopenia in patients receiving chemoradiotherapy for Glioblastoma. Int J Radiat Oncol Biol Phys 101(1):217–225
Choi SH, Seong J (2018) Strategic application of radiotherapy for hepatocellular carcinoma. Clin Mol Hepatol 24(2):114–134
Byun HK, Kim HJ, Im YR, Kim DY, Han KH, Seong JS (2019) Dose escalation by intensity modulated radiotherapy in liver-directed concurrent chemoradiotherapy for locally advanced BCLC stage C hepatocellular carcinoma. Radiother Oncol 133:1–8
National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers 2016; Version 2. 2016.
Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M (2015) Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 35(9):2155–2166
Liu J, Zhao Q, Deng W, Lu J, Xu X, Wang R, Li X, Yue J (2017) Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol 12(1):90
Falcke SE, Ruhle PF, Deloch L, Fietkau R, Frey B, Gaipl US (2018) Clinically Relevant Radiation Exposure Differentially Impacts Forms of Cell Death in Human Cells of the Innate and Adaptive Immune System. Int J Mol Sci 19(11):3574
Heylmann D, Badura J, Becker H, Fahrer J, Kaina B (2018) Sensitivity of CD3/CD28-stimulated versus non-stimulated lymphocytes to ionizing radiation and genotoxic anticancer drugs: key role of ATM in the differential radiation response. Cell Death Dis 9. https://doi.org/10.1038/s41419-018-1095-7
Belka C, Ottinger H, Kreuzfelder E, Weinmann M, Lindemann M, Lepple-Wienhues A, Budach W, Grosse-Wilde H, Bamberg M (1999) Impact of localized radiotherapy on blood immune cells counts and function in humans. Radiother Oncol 50(2):199–204
Ruhle PF, Fietkau R, Gaipl US, Frey B (2016) Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry. Int J Mol Sci 17(8):1316
Meyer KK (1970) Radiation-induced lymphocyte-immune deficiency. A factor in the increased visceral metastases and decreased hormonal responsiveness of breast cancer. Arch Surg 101(2):114–121
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348(3):203–213
Schumacher K, Haensch W, Roefzaad C, Schlag PM (2001) Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 61(10):3932–3936
Marrogi AJ, Munshi A, Merogi AJ, Ohadike Y, El-Habashi A, Marrogi OL, Freeman SM (1997) Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer 74(5):492–501
Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M (2002) Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer 95(3):588–595
Dawson LA, Eccles C, Craig T (2006) Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol 45(7):856–864
Feng M, Ben-Josef E (2011) Radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol 21(4):271–277
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling THR, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela CC, Lang L, Neely J, Tang H, Dastani HB, Melero I (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389(10088):2492–2502
Ruckert M, Deloch L, Fietkau R, Frey B, Hecht M, Gaipl US (2018) Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther Onkol 194(6):509–519
Mackall CL, Fry TJ, Gress RE (2011) Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol 11(5):330–342
Ellsworth S, Balmanoukian A, Kos F, Nirschl CJ, Nirschl TR, Grossman SA, Luznik L, Drake CG (2014) Sustained CD4(+) T cell-driven lymphopenia without a compensatory IL-7/IL-15 response among high-grade glioma patients treated with radiation and temozolomide. Oncoimmunology 3(1):e27357
Sudo T, Nishikawa S, Ohno N, Akiyama N, Tamakoshi M, Yoshida H, Nishikawa SI (1993) Expression and Function of the Interleukin-7 Receptor in Murine Lymphocytes. Proc Natl Acad Sci USA 90(19):9125–9129
Anon. (2016) Study of the Effect IL 7/NT-I7 on CD4 Counts in Patients With High Grade Gliomas. https://clinicaltrials.gov/ct2/show/NCT02659800?cond=IL-7+high+grade+glioma&rank=1
Funding
This study was supported by National Nuclear R&D Program through a National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (Grant number; 2017070426)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H.K. Byun, N. Kim, S. Park and J. Seong declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants performed by any of the authors.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Byun, H.K., Kim, N., Park, S. et al. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol 195, 1007–1017 (2019). https://doi.org/10.1007/s00066-019-01462-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-019-01462-5