Abstract
Purpose
To evaluate local recurrence in younger men treated with low-dose-rate (LDR) 125I brachytherapy (BT) for localized prostate cancer.
Patients and methods
A total of 192 patients (≤65-years-old) were treated with LDR 125I-BT ± hormone therapy. Local failure was defined as any prostate-specific antigen (PSA) rise leading to salvage treatment or biochemical failure according to the Phoenix definition. A bounce was defined as a rise in the nadir of ≥0.2 ng/mL followed by spontaneous return. Proportions were compared using Fisher’s exact tests; continuous variables using the unpaired t-test or its non-parametric equivalent. Cox proportional hazards models were applied for multivariable survival analysis.
Results
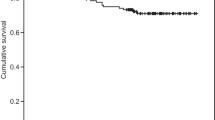
Median follow-up was 66 months. The 5‑year local recurrence-free survival was 96.1%. Biopsy-proven local recurrence developed in 13 patients, 4 had a Phoenix-defined recurrence at the last follow-up. Androgen deprivation therapy was started in 1 patient without proven recurrence. Univariable risk factors for local recurrence were: at least 50% positive biopsies, intermediate risk, treatment with neoadjuvant hormone therapy, low preimplantation volume receiving 100% of the prescribed dose, and no bounce development. Hormone-naïve patients not attaining a PSA value <0.5 ng/mL during follow-up also had a higher risk of local recurrences. Cox regression demonstrated that the variables “at least 50% positive biopsies” and “bounce” significantly impacted local failure (hazard ratio, HR 1.02 and 11.59, respectively). A bounce developed in 70 patients (36%). Younger patients and those treated with a lower activity per volume had a higher chance of developing a bounce in the Cox model (HR 0.99 and 0.04, respectively).
Conclusion
For younger men, LDR BT is a valid primary curative treatment option in low-risk and is to consider in intermediate-risk localized prostate cancer.
Zusammenfassung
Ziel
Bestimmung der Lokalrezidivrate bei jüngeren Patienten mit lokalisiertem Prostatakarzinom nach Low-Dose-Rate-(LDR-)Brachytherapie (BT) mit 125Iod-Seed-Implantaten.
Patienten und Methoden
Mit LDR-125Iod-BT ± Hormontherapie wurden 192 Patienten (≤65 Jahren) behandelt. Als Lokalrezidiv galt ein PSA-Anstieg, der zur Salvage-Therapie führte, oder ein biochemisches Rezidiv nach Phoenix-Definition. Als PSA-Wiederanstieg war eine Nadir-Erhöhung ≥0,2 ng/ml, gefolgt von spontaner Rückgang definiert. Verhältnisse wurden mit dem exakten Fisher-Test und kontinuierliche Variablen mit dem ungepaarten t-Test oder einer nichtparametrischen Methode verglichen. Mit multivariater Cox-Regressionsanalyse wurde der Einfluss von Kofaktoren untersucht.
Ergebnisse
Das mediane Follow-up betrug 66 Monate. Das lokalrezidivfreie 5‑Jahres-Überleben betrug 96,1 %. Ein histologisch nachgewiesenes Lokalrezidiv entwickelten 13 Patienten; ein Rezidiv nach Phoenix hatten bei der letzten Untersuchung 4 Patienten. Ein Patient ohne positive Prostatabiopsie bekam eine Hormontherapie. Univariate Risikofaktoren für ein Lokalrezidiv waren: mindestens 50 % positive Biopsien, intermediäres Risiko, Behandlung mit neoadjuvanter Hormontherapie, geringe Prostatavolumenabdeckung mit 100 % der vorgeschriebenen Dosis und kein PSA-Wiederanstieg. Hormonnaive Patienten, die keinen PSA-Wert <0,5 ng/ml im Follow-up erzielten, hatten ein höheres Lokalrezidivrisiko. Die Cox-Regression ergab, dass „mindestens 50 % positive Biopsien“ und „kein PSA-Wiederanstieg“ die lokale Kontrolle signifikant beeinflussen (Hazard Ratio [HR] je 1,02 und 11,59). Einen Wiederanstieg zeigten 70 Patienten (36 %). Jüngere Patienten und jene, die mit einer geringeren Aktivität pro Volumen behandelt wurden, hatten ein höheres Risiko für einen Wiederanstieg (je HR 0,99 und 0,04).
Schlussfolgerung
LDR-BT ist eine effektive kurative Behandlungsmethode für junge Männer mit Low-risk-Prostatakarzinom und ist auch bei intermediärem Risiko zu erwägen.

Similar content being viewed by others
References
National Comprehensive Cancer Network (2016) Prostate Cancer, practice guidelines in oncology v.3.2016.
Grimm P, Billiet I, Bostwick D et al (2012) Comparative analysis of prostate-specific antigen free survival outcomes for patients with low, intermediate and high risk prostate cancer treatment by radical therapy. Results from the Prostate Cancer Results Study Group. BJU Int 109(suppl 1):22–29
Chao MW, Grimm P, Yaxley J, Jagavkar R, Ng M, Lawrentschuk N (2015) Brachytherapy: state-of-the-art radiotherapy in prostate cancer. BJU Int 116(suppl3):80–88
Loblaw et al (2013) Which is the best radiation treatment for low risk prostate cancer? A comparison of stereotactic body radiotherapy, standard external beam radiotherapy or low dose rate brachytherapy. Eur J Cancer 49(Suppl2):S682, p 85. doi:10.1016/S0959-8049(13)70064-9.
Bechis SK, Carroll PR, Cooperberg MR (2011) Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol 29(2):235–241
Yamada Y, Bhatia S, Zaider M et al (2006) Favorable clinical outcomes of three-dimensional computer-optimized high-dose-rate prostate brachytherapy in the management of localized prostate cancer. Brachytherapy 5(3):157–164
Kimura T, Kido M, Miki K et al (2014) Mid-term outcome of permanent prostate iodine-125 brachytherapy in Japanese patients. Int J Urol 21(5):473–478
Alibhai SM, Krahn MD, Cohen MM et al (2004) Is there age bias in the treatment of localized prostate carcinoma? Cancer 100(1):72–81
Merglen A, Schmidlin F, Fioretta G et al (2007) Short- and long-term mortality with localized prostate cancer. Arch Intern Med 167(18):1944–1950
Critz FA, Williams WH, Levinson AK et al (2003) Prostate specific antigen bounce after simultaneous irradiation for prostate cancer: the relationship to patient age. J Urol 170(5):1864–1867
D’Amico AV, Whittington R, Malkowicz SB et al (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280(11):969–974
Roach M 3rd, Hanks G, Thames H Jr et al (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65(4):965–974
Mazeron R, Bajard A, Montbarbon X et al (2012) Permanent 125I-seed prostate brachytherapy: early prostate specific antigen value as a predictor of PSA bounce occurrence. Radiat Oncol 7:46–57
Burri RJ, Ho AY, Forsythe K et al (2010) Young men have equivalent biochemical outcomes compared with older men after treatment with brachytherapy for prostate cancer. Int J Radiat Oncol Biol Phys 77(5):1315–1321
Merrick GS, Butler WM, Wallner KE et al (2004) Permanent interstitial brachytherapy in younger patients with clinically organ-confined prostate cancer. Urology 64(4):754–759
Shapiro EY, Rais-Bahrami S, Morgenstern C et al (2009) Long-term outcomes in younger men following permanent prostate brachytherapy. J Urol 181(4):1665–1671
Peschel RE, Khan A, Colberg H, Wilson LD (2006) The effect of age on prostate implantation results. Cancer J 12(4):305–308
Gómez-Iturriaga Piña A, Crook J, Borg J et al (2010) Median 5 year follow-up of 125iodine brachytherapy as monotherapy in men aged <or=55 years with favorable prostate cancer. Urology 75(6):1412–1416
Kollmeier MA, Fidaleo A, Pei X et al (2013) Favourable long-term outcomes with brachytherapy-based regimens in men ≤60 years with clinically localized prostate cancer. BJU Int 111(8):1231–1236
Tran AT, Mandall P, Swindell R et al (2013) Biochemical outcomes for patients with intermediate risk prostate cancer treated with I‑125 interstitial brachytherapy monotherapy. Radiother Oncol 109(2):235–240
Cosset JM, Flam T, Thiounn N et al (2008) Selecting patients for exclusive permanent implant prostate brachytherapy: the experience of the Paris Institut Curie/Cochin Hospital/Necker Hospital group on 809 patients. Int J Radiat Oncol Biol Phys 71(4):1042–4018
Hinnen KA, Battermann JJ, van Roermund JG et al (2010) Long-term biochemical and survival outcome of 921 patients treated with I‑125 permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 76(5):1433–1438
Herbert C, Morris WJ, Keyes M et al (2012) Outcomes following iodine-125 brachytherapy in patients with Gleason 7, intermediate risk prostate cancer: a population-based cohort study. Radiother Oncol 103(2):228–232
Henry AM, Al-Qaisieh B, Gould K et al (2010) Outcomes following iodine-125 monotherapy for localized prostate cancer: the results of leeds 10-year single-center brachytherapy experience. Int J Radiat Oncol Biol Phys 76(1):50–56
Hayashi N, Izumi K, Sano F et al (2015) Ten-year outcomes of I125 low-dose-rate brachytherapy for clinically localized prostate cancer: a single-institution experience in Japan. World J Urol 33(10):1519–1526
Wilson C, Waterhouse D, Lane S et al (2016) Ten-year outcomes using low dose rate brachytherapy for localised prostate cancer: an update of the first Australian experience. J Med Imaging Radiat Oncol 60(4):531–538
Caloglu M, Ciezki J (2009) Prostate-specific antigen bounce after prostate brachytherapy: review of a confusing phenomenon. Urology 74(6):1183–1190
Stock RG, Stone NN, Cesaretti JA (2003) Prostate-specific antigen bounce after prostate seed implantation for localized prostate cancer: descriptions and implications. Int J Radiat Oncol Biol Phys 56(2):448–453
Engeler DS, Schwab C, Thöni AF et al (2015) PSA bounce after 125I-brachytherapy for prostate cancer as a favorable prognosticator. Strahlenther Onkol 191:787–791
Wolff R, Ryder S, Bossi A et al (2015) A systematic review of randomized controlled trials of radiotherapy for localized prostate cancer. Eur J Cancer 51:2345–2367
Zelefsky M, Poon B, Eastham J, Vickers A, Pei X, Scardino P (2016) Longitudinal assessment of quality of life after surgery, conformal brachytherapy, and intensity-modulated radiation therapy for prostate cancer. Radiother Oncol 118:85–91
Acknowledgements
The authors acknowledge gratefully F. Ameye, B. Bamelis, M. D’Hoedt, S. Huybrechts, W. Kerkhaert, K. Lesage, P. Schoonooghe, D. Vandervaeren, P. Verleyen, P. Vossaert, and P. Werbrouck for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I. Kindts, K. Stellamans, I. Billiet, Hans Pottel, and A. Lambrecht declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Kindts, I., Stellamans, K., Billiet, I. et al. 125I brachytherapy in younger prostate cancer patients. Strahlenther Onkol 193, 707–713 (2017). https://doi.org/10.1007/s00066-017-1142-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1142-9