Abstract
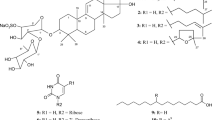
Biochemical investigation of bioactive compounds from the marine demospongiae Clathria (Thalysias) vulpina (Lamarck, 1814) (family Microcionidae) resulted in the isolation of two previously undescribed 22-membered macrocyclic lactone derivatives clathrolide A and B from its organic extract. Structural elucidation of the compounds were carried out using spectroscopic analysis. Clathrolide A exhibited greater antihypertensive (IC50 0.44 ± 0.02 mM), anti-inflammatory (IC50 0.74 ± 0.04 mM), anti-hyperglycemic (IC50 0.85 ± 0.01 mM), and antioxidant (IC50 0.70 ± 0.03 mM) activities compared to clathrolide B. Among various bioactivities, such as antihypertensive, anti-inflammatory, anti-hyperglycemic, and antioxidant properties, angiotensin converting enzyme attenuation potential of clathrolide A was found to be significantly greater, and comparable with the standard antihypertensive drug, captopril (IC50 0.36 ± 0.03 mM). Higher electronic properties (topological polar surface area 133.52) along with comparatively lesser docking score and binding energy of clathrolide A with the aminoacyl residues of angiotensin-I converting enzyme (−12.38 and −12.91 kcal/mol, respectively) recognized its prospective inhibitory property against the enzyme catalyzing the rate-limiting step to form the vasoconstrictor angiotensin-II.





Similar content being viewed by others
Data availability
The chromatographic and spectroscopic spectral data are included as Supplementary item.
References
Murray BA, FitzGerald RJ. Angiotensin converting enzyme inhibitory peptides derived from food proteins: biochemistry, bioactivity and production. Curr Pharm Des. 2007;13:773–91. https://doi.org/10.2174/138161207780363068.
Tedla YG, Bautista LE. Drug side effect symptoms and adherence to antihypertensive medication. Am J Hypertens. 2016;29:772–9. https://doi.org/10.1093/ajh/hpv185.
Suleria HA, Osborne S, Masci P, Gobe G. Marine-based nutraceuticals: an innovative trend in the food and supplement industries. Mar Drugs. 2015;13:6336–51. https://doi.org/10.3390/md13106336.
Montaser R, Luesch H. Marine natural products: a new wave of drugs? Future Med Chem. 2011;3:1475–89. https://doi.org/10.3390/md13106336.
Chakraborty K, Francis P. Procerolides A-B from Microcionidae marine sponge Clathria procera: anti-inflammatory macrocylic lactones with selective cyclooxygenase-2 attenuation properties. Bioorg Chem. 2021;109:104663. https://doi.org/10.1016/j.bioorg.2021.104663.
Chakraborty K, Antony T. Salicornolides A-C from Gracilaria salicornia attenuate pro-inflammatory 5-lipoxygense: prospective natural anti-inflammatory leads. Phytochemistry. 2020;172:112259. https://doi.org/10.1016/j.phytochem.2020.112259.
Francis P, Chakraborty K. Stomopnolides A-B from echinoidea sea urchin Stomopneustes variolaris: prospective natural anti-inflammatory leads attenuate pro-inflammatory 5-lipoxygenase. Nat Prod Res. 2019 Nov 29:1-13. https://doi.org/10.1080/14786419.2019.1696332. Epub ahead of print. PMID: 31782657.
Chakraborty K, Krishnan S, Joy M. Macrocyclic lactones from seafood Amphioctopus neglectus: newly described natural leads to attenuate angiotensin-II induced cardiac hypertrophy. Biomed Pharmacother. 2019;110:155–67. https://doi.org/10.1016/j.biopha.2018.11.034.
Chakraborty K, Francis P. Stomopneulactone D from long-spined sea urchin Stomopneustes variolaris: anti-inflammatory macrocylic lactone attenuates cyclooxygenase-2 expression in lipopolysaccharide-Activated macrophages. Bioorg Chem. 2020;103:104140. https://doi.org/10.1016/j.bioorg.2020.104140.
Salas S, Chakraborty K. An unreported polyether macrocyclic lactone with antioxidative and anti-lipoxygenase activities from the Babylonidae gastropod mollusc Babylonia spirata. Med Chem Res. 2018;27:2446–53. https://doi.org/10.1007/s00044-018-2248-z.
Yan S, Ci X, Chen N, Chen C, Li X, Chu X, et al. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm Res. 2011;60:589–96. https://doi.org/10.1007/s00011-011-0307-8.
Remko M. Acidity, lipophilicity, solubility, absorption, and polar surface area of some ACE inhibitors. Chem Pap. 2007;61:133–41. https://doi.org/10.2478/s11696-007-0010-y.
Ebada SS, Lin W, Proksch P. Bioactive sesterterpenes and triterpenes from marine sponges: occurrence and pharmacological significance. Mar Drugs. 2010;8:313–46. https://doi.org/10.3390/md8020313.
Molinski TF. Cyclic azole-homologated peptides from marine sponges. Org Biomol Chem. 2017;16:21–9. https://doi.org/10.1039/c7ob02628e.
Aiello A, Fattorusso E, Menna M. Steroids from sponges: recent reports. Steroid. 1999;64:687–714. https://doi.org/10.1016/S0039-128X(99)00032-X.
Francis P, Chakraborty K. Anti-inflammatory scalarane-type sesterterpenes, erectascalaranes A–B, from the marine sponge Hyrtios erectus attenuate pro-inflammatory cyclooxygenase-2 and 5-lipoxygenase. Med Chem Res. 2021;30:886–96. https://doi.org/10.1007/s00044-020-02682-6.
Pettit GR, Cichacz ZA, Gao F, Boyd MR, Schmidt JM. Isolation and structure of the cancer cell growth inhibitor dictyostatin 1. J Chem Soc Chem Commun. 1994;9:1111–2. https://doi.org/10.1039/C39940001111.
Ko S-C, Jang J, Ye B-R, Kim M-S, Choi I-W, Park W-S, et al. Purification and molecular docking study of angiotensin I-converting enzyme (ACE) inhibitory peptides from hydrolysates of marine sponge Stylotella aurantium. Process Biochem. 2017;54:180–7. https://doi.org/10.1016/j.procbio.2016.12.023.
Woo JK, Ha TKQ, Oh DC, Oh WK, Oh KB, Shin J. Polyoxygenated steroids from the sponge Clathria gombawuiensis. J Nat Prod. 2017;80:3224–33. https://doi.org/10.1021/acs.jnatprod.7b00651.
Capon RJ, Miller M, Rooney F, Mirabilin G. A new alkaloid from a southern Australian marine sponge, Clathria species. J Nat Prod. 2001;64:643–4. https://doi.org/10.1021/np000564g.
Davis RA, Mangalindan GC, Bojo ZP, Antemano RR, Rodriguez NO, Concepcion GP, et al. Microcionamides A and B, bioactive peptides from the Philippine sponge Clathria (Thalysias) abietina. J Org Chem. 2004;69:4170–6. https://doi.org/10.1021/jo040129h.
Joy M, Chakraborty K. Biogenic antioxidative and anti-inflammatory aryl polyketides from the venerid bivalve clam Paphia malabarica. Food Chem. 2017;237:169–80. https://doi.org/10.1016/j.foodchem.2017.05.087.
Antony T, Chakraborty K. Xenicanes attenuate pro-inflammatory 5-lipoxygenase: prospective natural anti-inflammatory leads from intertidal brown seaweed Padina tetrastromatica. Med Chem Res. 2019;28:597–607. https://doi.org/10.1007/s00044-019-02322-8.
Li G-H, Le G-W, Shi Y-H, Shrestha S. Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res. 2004;24:469–86. https://doi.org/10.1016/j.nutres.2003.10.014.
De Giusti VC, Caldiz CI, Ennis IL, Pérez NG, Cingolani HE, Aiello EA. Mitochondrial reactive oxygen species (ROS) as signaling molecules of intracellular pathways triggered by the cardiac renin-angiotensin II-aldosterone system (RAAS). Front Physiol. 2013;4:126. https://doi.org/10.3389/fphys.2013.00126.
Baradaran A, Nasri H, Rafieian-Kopaei M. Oxidative stress and hypertension: possibility of hypertension therapy with antioxidants. J Res Med Sci. 2014;19:358–67.
Vajragupta O, Boonchoong P, Berliner LJ. Manganese complexes of curcumin analogues: evaluation of hydroxyl radical scavenging ability, superoxide dismutase activity and stability towards hydrolysis. Free Radic Res. 2004;38:303–14.
Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–60. https://doi.org/10.1111/j.1582-4934.2009.00897.x.
Mazaleuskaya LL, Theken KN, Gong L, Thorn CF, FitzGerald GA, Altman RB, et al. Pharm GKB summary: ibuprofen pathways. Pharmacogenet Genomics.2015;25:96–106. https://doi.org/10.1097/FPC.0000000000000113.
Maneesh A, Chakraborty K, Makkar F. Pharmacological activities of brown seaweed Sargassum wightii (Family Sargassaceae) using differentin vitromodels. Int J Food Prop. 2017;20:931–45. https://doi.org/10.1080/10942912.2016.1189434.
Truscheit E, Frommer W, Junge B, Müller L, Schmidt DD, Wingender W. Chemistry and biochemistry of microbial α-glucosidase inhibitors. Angew Chem Int. 1981;20:744–61.
Matos MJ, Pérez-Cruz F, Vazquez-Rodriguez S, Uriarte E, Santana L, Borge F, et al. Remarkable antioxidant properties of a series of hydroxy-3-arylcoumarins. Bioorg Med Chem. 2013;21:3900–6. https://doi.org/10.1016/j.bmc.2013.04.015.
Lipinski C, Hopkins A. Navigating chemical space for biology and medicine. Nature. 2004;432:855–61. https://doi.org/10.1038/nature03193.
Maneesh A, Chakraborty K. Previously undescribed fridooleanenes and oxygenated labdanes from the brown seaweed Sargassum wightii and their protein tyrosine phosphatase-1B inhibitory activity. Phytochemistry. 2017;144:19–32. https://doi.org/10.1016/j.phytochem.2017.08.011.
Maneesh A, Chakraborty K. Unprecedented antioxidative and anti-inflammatory aryl polyketides from the brown seaweed Sargassum wightii. Food Res Int. 2017;100:640–9. https://doi.org/10.1016/j.foodres.2017.07.006.
Chakraborty K, Maneesh A, Makkar F. Antioxidant activity of brown seaweeds. J Aquat Food Prod T. 2017;26:406–19. https://doi.org/10.1080/10498850.2016.1201711.
Maneesh A, Chakraborty K. Previously undescribed antioxidative O-heterocyclic angiotensin converting enzyme inhibitors from the intertidal seaweed Sargassum wightii as potential antihypertensives. Food Res Int. 2018;113:474–86. https://doi.org/10.1016/j.foodres.2018.07.035.
Natesh R, Schwager SLU, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature. 2003;421:551–4. https://doi.org/10.1038/nature01370.
Acknowledgements
The authors gratefully acknowledge the funding by the Indian Council of Agricultural Research (ICAR, New Delhi, India) under Central Marine Fisheries Research Institute (ICAR-CMFRI) project “Development of Bioactive Pharmacophores from Marine Organisms” (MBT/HLT/SUB23). The authors are grateful to the Director, ICAR-CMFRI, and Head, Marine Biotechnology Division of ICAR-CMFRI for facilitating the research activities. The authors are thankful to the Chairman, Department of Chemistry, Mangalore University (Karnataka, India) for providing with necessary support.
Author information
Authors and Affiliations
Contributions
PF and KC designed research, conducted experiments, and analyzed data. KC acquired funds and conceptualized the work. PF drafted the manuscript. KC reviewed and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Francis, P., Chakraborty, K. Clathrolides A–B: previously undescribed macrocylic lactones from marine demosponge Clathria (Thalysias) vulpina (Lamarck, 1814) as potential antihypertensive leads attenuating angiotensin converting enzyme. Med Chem Res 30, 1438–1451 (2021). https://doi.org/10.1007/s00044-021-02743-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-021-02743-4