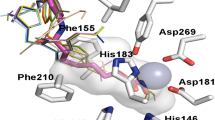
Abstract
Molecular hybridization has become a new promising way to treat multifactorial diseases with a single compound that acts on multiple targets. The combination of several functional pharmacophore groups in one molecule can lead to a stronger therapeutic effect due to the ability to bind to several targets and possible synergistic interactions. The concept of multifunctional agents is being actively developed and has already produced some encouraging results. The quinazoline cycle and hydroxamic acids are unique pharmacophore groups that contribute to the structure of drug agents widely used in medical chemistry. The combination of these pharmacophores in one molecule leads to promising new compounds, which has been confirmed by many experimental studies in published literature across the world. Hybrid compounds of hydroxamic acids and the quinazoline cycle are a potential basis for the development of effective drugs used in the complex treatment of oncological, infectious and neurological diseases. This review provides information on the most significant developments in this area and discusses the bioactivity of important agents. Compounds with both linear hydroxamic acids and cyclic acids in which a hydroxamate group is integrated in the quinazoline ring are also covered in this review.
















Similar content being viewed by others
References
Abzianidze VV, Prokofieva DS, Chisty LA, Bolshakova KP, Berestetskiy AO, Panikorovskii TL, Bogachenkov AS, Holder AA (2015) Synthesis of natural phaeosphaeride A derivatives and an in-vitro evaluation of their anti-cancer potential. Bioorg Med Chem Lett 25:5566–5569
Apfel C, Banner DW, Bur D, Dietz M, Hubschwerlen C, Locher H, Marlin F, Masciadri R, Pirson W, Stalder H (2001) 2-(2-Oxo-1,4-dihydro-2H-quinazolin-3-yl)- and 2-(2,2-Dioxo-1,4-dihydro-2H-2λ6-benzo[1,2,6]thiadiazin-3-yl)-N-hydroxy-acetamides as potent and selective peptide deformylase inhibitors. J Med Chem 44:1847–1852
Arrighetti N, Corno C, Gatti L (2015) Drug combinations with HDAC inhibitors in antitumor therapy. Crit Rev Oncog 20:83–117
Asif M (2014) Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives. Int J Med Chem 2014:1–27
Barlaam B, Bird TG, Lambert-van der Brempt C, Campbell D, Foster SJ, Maciewicz R (1999) New α-Substituted succinate-based hydroxamic acids as TNFα convertase inhibitors. J Med Chem 42:4890–4908
Beckers T, Mahboobi S, Sellmer A, Winkler M, Eichhorn E, Pongratz H, Maier T, Ciossek T, Baer T, Kelter G, Fiebig H, Schmidt M (2012) Chimerically designed HDAC- and tyrosine kinase inhibitors. A series of erlotinib hybrids as dual-selective inhibitors of EGFR, HER2 and histone deacetylases. MedChemComm 3:829
Bérubé G (2016) An overview of molecular hybrids in drug discovery. Expert Opin Drug Dis 11:281–305
Billamboz M, Suchaud V, Bailly F, Lion C, Andréola M-L, Christ F, Debyser Z, Cotelle P (2016) 2-hydroxyisoquinoline-1,3(2H,4H)-diones (HIDs) as human immunodeficiency virus type 1 integrase inhibitors: influence of the alkylcarboxamide substitution of position 4. Eur J Med Chem 117:256–268
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Disco 5:769–784
Bonola G, Sianesi E (1970) 2,3-Dihydro-4(1H)-quinazolinone derivatives. J Med Chem 13:329–332
Bratu M, Nuta DC, Caproiu MT, Missir AV, Limban C, Ileana C, Morusciag L (2014) New acylated derivatives of 2-methyl-4-oxo-quinazolin-3(4H)-yl-acetohydroxamic acid. Farmacia 62:664–673
Catarzi D, Lenzi O, Colotta V, Varano F, Poli D, Filacchioni G, Lingenhöhl K, Ofner S (2010) Pharmacological characterization of some selected 4,5-Dihydro-4-oxo-1,2,4-triazolo[1,5-a]quinoxaline-2-carboxylates and 3-Hydroxyquinazoline-2,4-diones as (S)-2-Amino-3-(3-hydroxy-5-methylisoxazol-4-yl)-propionic Acid Receptor Antagonists. Chem Pharm Bull 58:908–911
Chen J, Sang Z, Jiang Y, Yang C, He L (2018) Design, synthesis, and biological evaluation of quinazoline derivatives as dual HDAC1 and HDAC6 inhibitors for the treatment of cancer. Chem Biol Drug Des 93:232–241
Chollet A-M, Le Diguarher T, Murray L, Bertrand M, Tucker GC, Sabatini M, Pierré A, Atassi G, Bonnet J, Casara P (2001) General synthesis of α-substituted 3-bisaryloxy propionic acid derivatives as specific mmp inhibitors. Bioorg Med Chem Lett 11:295–299
Cianci C, Chung TDY, Meanwell N, Putz H, Hagen M, Colonno RJ, Krystal M (1996) Identification of N-Hydroxamic acid and N-Hydroxyimide compounds that inhibit the influenza virus polymerase. Antivir Chem Chemother 7:353–360
Colotta V, Catarzi D, Varano F, Calabri FR, Filacchioni G, Costagli C, Galli A (2004) 3-Hydroxy-quinazoline-2,4-dione as a useful scaffold to obtain selective Gly/NMDA and AMPA receptor antagonists. Bioorg Med Chem Lett 14:2345–2349
Colotta V, Catarzi D, Varano F, Lenzi O, Filacchioni G, Costagli C, Galli A, Ghelardini C, Galeotti N, Gratteri P, Sgrignani J, Deflorian F, Moro S (2006) Structural investigation of the 7-chloro-3-hydroxy-1H-quinazoline-2,4-dione scaffold to obtain AMPA and kainate receptor selective antagonists. Synthesis, pharmacological, and molecular modeling studies. J Med Chem 49:6015–6026
Colotta V, Lenzi O, Catarzi D, Varano F, Squarcialupi L, Costagli C, Galli A, Ghelardini C, Pugliese AM, Maraula G, Coppi E, Pellegrini-Giampietro DE, Pedata F, Sabbadin D, Moro S (2012) 3-Hydroxy-1H-quinazoline-2,4-dione derivatives as new antagonists at ionotropic glutamate receptors: molecular modeling and pharmacological studies. Eur J Med Chem 54:470–482
Deore RR, Chen GS, Chang P-T, Chern T-R, Lai S-Y, Chuang M-H, Lin JH, Kung FL, Chen CS, Chiou CT, Chern J-W (2012) Discovery of N-Arylalkyl-3-hydroxy-4-oxo-3,4-dihydroquinazolin-2-carboxamide derivatives as HCV NS5B polymerase inhibitors. ChemMedChem 7:850–860
Dikii IL, Kris’kiv OS, Chernikh VP, Shemchuk LA, Dubinina NV (2006) Study of antimicrobial activity of quinazolin-4-ones and heterocyclic derivatives. Visn Farmatsii 2:64–67
Ding C, Chen S, Zhang C, Hu G, Zhang W, Li L, Chen YZ, Tan C, Jiang Y (2017) Synthesis and investigation of novel 6-(1,2,3-triazol-4-yl)-4-aminoquinazolin derivatives possessing hydroxamic acid moiety for cancer therapy. Bioorg Med Chem 25:27–37
Falsini M, Squarcialupi L, Catarzi D, Varano F, Betti M, Di Cesare Mannelli L, Tenci B, Ghelardini C, Tanc M, Angeli A, Supuran CT, Colotta V (2017) 3-Hydroxy-1H-quinazoline-2,4-dione as a new scaffold to develop potent and selective inhibitors of the tumor-associated carbonic anhydrases IX and XII. J Med Chem 60:6428–6439
Fetisov VI, Kotov AV, Gordeev PB, Bachurin SO, Petrova LN, Luk’janov OA, Martynov IV(1999) Sintez i izuchenie vlijanija prozvodnyh 3-gidroksi-1,2-digidrohinolin-4-onanaglutamat-inducirovannyj zahvat 45Ca2+ sinaptosomami mozga krys [The synthesis and the study of influence of derivatives of 3-hydroxy-1,2-dihydroquinoline-4-one on the glutamate-induced 45Ca2+-uptake by rat brain synaptosomes]. Dokl Akad Nauk Rep Acad Sci 367:776–779
Fortin S, Bérubé G (2013) Advances in the development of hybrid anticancer drugs. Expert Opin Drug Dis 8:1029–1047
Ganesan A (2016) Multitarget drugs: an epigenetic epiphany. ChemMedChem 11:1227–1241
Giannini G, Battistuzzi G, Vignola D (2015) Hydroxamic acid based histone deacetylase inhibitors with confirmed activity against the malaria parasite. Bioorg Med Chem Lett 25:459–461
Grady RW, Bienen EJ, Clarkson AB (1986) P-alkyloxybenzhydroxamic acids, effective inhibitors of the trypanosome glycerol-3-phosphate oxidase. Mol Biochem Parasit 19:231–240
Gupta SP, Sharma A (2013) Hydroxamic acids. A unique family of chemicals with multiple biological activities. Gupta SP (ed.), Springer-Verlag, Berlin
He S, Dong G, Wang Z, Chen W, Huang Y, Li Z, Jiang Y, Liu N, Yao J, Miao Z, Zhang W, Sheng C (2015) Discovery of novel multiacting topoisomerase I/II and histone deacetylase inhibitors. ACS Med Chem Lett 6:239–243
Hemalatha K, Madhumitha G (2016) Synthetic strategy with representation on mechanistic pathway for the therapeutic applications of dihydroquinazolinones. Eur J Med Chem 123:596–630
Hesham HM, Lasheen DS, Abouzid KAM (2018) Chimeric HDAC inhibitors: comprehensive review on the HDAC-based strategies developed to combat cancer. Med Res Rev 38:2058–2109
Hider RC, Kong X (2010) Chemistry and biology of siderophores. Nat Prod Rep 27:637
Hieu DT, Anh DT, Hai P-T, Huong L-T-T, Park EJ, Choi JE, Kang JS, Dung PTP, Han SB, Nam N-H (2018a) Quinazoline-based hydroxamic acids: design, synthesis, and evaluation of histone deacetylase inhibitory effects and cytotoxicity. Chem Biodivers 15:e1800027
Hieu DT, Anh DT, Tuan NM, Hai P-T, Huong L-T-T, Kim J, Kang JS, Vu TK, Dung PTP, Han SB, Nam NH, Hoa N-D (2018b) Design, synthesis and evaluation of novel N -hydroxybenzamides/ N -hydroxypropenamides incorporating quinazolin-4(3H)-ones as histone deacetylase inhibitors and antitumor agents. Bioorg Chem 76:258–267
Ji M, Li Z, Lin Z, Chen L (2018) Antitumor activity of the novel HDAC inhibitor CUDC-101 combined with gemcitabine in pancreatic cancer. Am J Cancer Res 8:2402–2418
Jung R, Le JY, Wengenmayer F, Wolf E, Kramer M (1985) Mutagenicity studies of a carcinogenic nitrofuran and some analogs. Biochimica Acta 44:485–492
Kerru N, Singh P, Koorbanally N, Raj R, Kumar V (2017) Recent advances (2015-2016) in anticancer hybrids. Eur J Med Chem 142:179–212
Khan I, Zaib S, Batool S, Abbas N, Ashraf Z, Iqbal J, Saeed A (2016) Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: an update on the development of synthetic methods and pharmacological diversification. Bioorg Med Chem 24:2361–2381
Khohlov PS, Osipov VN, Krivenko VI, Zubairov MM, Roshhin AV, Batuev EA (2011) 2-(2,5-Dimethyl)pyrazolyl-3-hydroxy-4(3H)-quinazolinone possessing antiviral, antibacterial and fungicidal activity and its production method. RU Patent RU2451683, 10 Mar 2011
Khohlov PS, Pavlova VV, Shumova TB, Poljanskaja SM (2005) 3-Hydroxy-2-thioxo-4(3h)-quinazolinone possessing fungicide and growth-regulating property and method for it preparing. RU Patent RU2275362, 27 Oct 2005
Khokhlov PS, Osipov VN, Roshchin AV (2011) 3-Hydroxy- and 3-alkoxy-2-sulfanylquinazolin-4(3H)-ones: synthesis and reactions with alkylating and acylating agents. Russ Chem Bull 60:153–156
Kobayashi K, Kobayashi Y, Nakamura M, Tamura O, Kogen H (2015) Establishment of relative and absolute configurations of Phaeosphaeride A: total synthesis of ent-Phaeosphaeride A. J Org Chem 80:1243–1248
Kotov AV, Zakharychev VV, Smirnov AG, Fetisov VI, Gordeev PB, Luk’yanov OA, Chimishkyan AL, Martynov IV (2001) The fungicidal activity of the 3-hydroxy-1,2,3,4-tetrahydroquinazoline-4-one derivatives and simulation of the structure-activity dependence. Dokl Biochem Biophysics 381:412–414
Lavi O (2015) Redundancy: a critical obstacle to improving cancer therapy. Cancer Res 75:808–812
Li Y, Ganesh T, Diebold BA, Zhu Y, McCoy JW, Smith SME, Sun A, Lambeth JD (2015) Thioxo-dihydroquinazolin-one compounds as novel inhibitors of myeloperoxidase. ACS Med Chem Lett 6:1047–1052
Lou B, Yang K (2003) Molecular diversity of hydroxamic acids. Part II: potential therapeutic applications. Mini-Rev Med Chem 3:609–620
Lu W, Baig IA, Sun H-J, Cui C-J, Guo R, Jung I-P, Wang J-G (2015) Synthesis, crystal structure and biological evaluation of substituted quinazolinone benzoates as novel antituberculosis agents targeting acetohydroxyacid synthase. Eur J Med Chem 94:298–305
Mahboobi S, Sellmer A, Winkler M, Eichhorn E, Pongratz H, Ciossek T, Baer T, Maier T, Beckers T (2010) Novel chimeric histone deacetylase inhibitors: a series of lapatinib hybrides as potent inhibitors of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and histone deacetylase activity. J Med Chem 53:8546–8555
Maloney KN, Hao W, Xu J, Gibbons J, Hucul J, Roll D, Brady SF, Schroeder FC, Clardy J (2006) Phaeosphaeride A, an inhibitor of STAT3-dependent signaling isolated from an Endophytic fungus. Org Lett 8:4067–4070
Manal M, Chandrasekar MJN, Gomathi Priya J, Nanjan MJ (2016) Inhibitors of histone deacetylase as antitumor agents: a critical review. Bioorg Chem 67:18–42
Meng Q, Li F, Jiang S, Li Z (2013) Novel 64Cu-labeled CUDC-101 for in vivo pet imaging of histone deacetylases. ACS Med Chem Lett 4:858–862
Mottamal M, Zheng S, Huang T, Wang G (2015) Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 20:3898–3941
Muri E, Nieto M, Sindelar R, Williamson J (2002) Hydroxamic acids as pharmacological agents. Curr Med Chem 9:1631–1653
Musso L, Dallavalle S, Zunino F (2015) Perspectives in the development of hybrid bifunctional antitumour agents. Biochem Pharm 96:297–305
Mutule I, Borovika D, Rozenberga E, Romanchikova N, Zalubovskis R, Shestakova I, Trapencieris P (2014) 5-Membered cyclic hydroxamic acids as HDAC inhibitors. J Enzym Inhib Med Chem 30:216–223
Nara H, Kaieda A, Sato K, Naito T, Mototani H, Oki H, Yamamoto Yl, Kuno H, Santou T, Kanzaki N, Terauchi J, Uchikawa O, Kori M (2017) Discovery of novel, highly potent, and selective matrix metalloproteinase (MMP)-13 inhibitors with a 1,2,4-triazol-3-yl Moiety as a zinc binding group using a structure-based design approach. J Med Chem 60:608–626
Nara H, Sato K, Kaieda A, Oki H, Kuno H, Santou T, Kanzaki N, Terauchi J, Uchikawa O, Kori M (2016) Design, synthesis, and biological activity of novel, potent, and highly selective fused pyrimidine-2-carboxamide-4-one-based matrix metalloproteinase (MMP)-13 zinc-binding inhibitors. Bioorg Med Chem 24:6149–6165
Nepali K, Sharma S, Sharma M, Bedi PMS, Dhar KL (2014) Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem 77:422–487
Niemeyer HM (1988) Hydroxamic acids (4-hydroxy-1,4-benzoxazin-3-ones), defence chemicals in the gramineae. Phytochemistry 27:3349–3358
Niemeyer HM (2009) Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: key defense chemicals of cereals. J Agric Food Chem 57:1677–1696
Papavassiliou KA, Papavassiliou AG (2013) Histone deacetylases inhibitors: conjugation to other anti-tumour pharmacophores provides novel tools for cancer treatment. Expert Opin Inv Drug 23:291–294
Peng F-W, Wu T-T, Ren Z-W, Xue J-Y, Shi L (2015) Hybrids from 4-anilinoquinazoline and hydroxamic acid as dual inhibitors of vascular endothelial growth factor receptor-2 and histone deacetylase. Bioorg Med Chem Lett 25:5137–5141
Peng F-W, Xuan J, Wu T-T, Xue J-Y, Ren Z-W, Liu D-K, Wang XQ, Chen XH, Zhang JW, Xu YG, Shi L (2016) Design, synthesis and biological evaluation of N-phenylquinazolin-4-amine hybrids as dual inhibitors of VEGFR-2 and HDAC. Eur J Med Chem 109:1–12
Pryde DC, Webster R, Butler SL, Murray EJ, Whitby K, Pickford C, Westby M, Palmer MJ, Bull DJ, Vuong H, Blakemore DC, Stead D, Ashcroft C, Gardner I, Bru C, Cheung W-Y, Roberts IO, Mortone J, Bissell RA (2013) Discovery of an HIV integrase inhibitor with an excellent resistance profile. MedChemComm 4:709
Qian Ch, Cai X, Zhai H (2009) Antiproliferative agents containing a zinc binding moiety. PCT International Application No. WO 2009036057 A1
Qiu X, Xiao X, Li N, Li Y (2017) Histone deacetylases inhibitors (HDACis) as novel therapeutic application in various clinical diseases. Prog Neuro-Psychoph 72:60–72
Rani R, Granchi C (2015) Bioactive heterocycles containing endocyclic N-hydroxy groups. Eur J Med Chem 97:505–524
Rao M, Valentini D, Zumla A, Maeurer M (2018) Evaluation of the efficacy of valproic acid and suberoylanilide hydroxamic acid (vorinostat) in enhancing the effects of first-line tuberculosis drugs against intracellular Mycobacterium tuberculosis. Int J Infect Dis 69:78–84
Rao MJ (1992) Antifungal potential of binary and mixed-ligand complexes of N,2′-diphenyl acetohydroxamic acid. J Inorg Biochem 46:207–214
Schobert R, Biersack B (2017) Multimodal HDAC inhibitors with improved anticancer activity Curr Cancer Drug Tar 18:39–56
Seo S-Y (2012) Multi-targeted hybrids based on HDAC inhibitors for anti-cancer drug discovery. Arch Pharm Res 35:197–200
Shagufta S, Ahmad I (2017) An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. MedChemComm 8:871–885
Shao M, He L, Zheng L, Huang L, Zhou Y, Wang T, Chen Y, Shen M, Wang F, Yang Z, Chen L (2017) Structure-based design, synthesis and in vitro antiproliferative effects studies of novel dual BRD4/HDAC inhibitors. Bioorg Med Chem Lett 27:4051–4055
Shimizu T, LoRusso PM, Papadopoulos KP, Patnaik A, Beeram M, Smith LS, Rasco DW, Mays TA, Chambers G, Ma A, Wang J, Laliberte R, Voi M, Tolcher AW (2014) Phase I first-in-human study of CUDC-101, a multi-targeted inhibitor of HDACs, EGFR and HER2 in patients with advanced solid tumors. Clin Cancer Res 20:5032–5040
Sun H, Mediwala SN, Szafran AT, Mancini MA, Marcelli M (2016) CUDC-101, a novel inhibitor of full-length androgen receptor (flAR) and androgen receptor variant 7 (AR-V7) activity: mechanism of action and in vivo efficacy. Hormones Cancer 7:196–210
Suraweera A, O’Byrne KJ, Richard DJ (2018) Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi Front Oncol 8:1–15
Tang J, Vernekar SKV, Chen Y-L, Miller L, Huber AD, Myshakina N, Wang Z (2017) Synthesis, biological evaluation and molecular modeling of 2-Hydroxyisoquinoline-1,3-dione analogues as inhibitors of HIV reverse transcriptase associated ribonuclease H and polymerase. Eur J Med Chem 133:85–96
Tran TP, Ellsworth EL, Stier MA, Domagala JM, Hollis Showalter HD, Gracheck SJ, Singh R (2004) Synthesis and structural-activity relationships of 3-hydroxyquinazoline-2,4-dione antibacterial agents. Bioorg Med Chem Lett 14:4405–4409
Tumey LN, Bom D, Huck B, Gleason E, Wang J, Silver D, Bennani YL (2005) The identification and optimization of a N-hydroxy urea series of flap endonuclease 1 inhibitors. Bioorg Med Chem Lett 15:277–281
Wang D, Zhu X, Cui C, Dong M, Jiang H, Li Z, Wang J-G (2013) Discovery of novel acetohydroxyacid synthase inhibitors as active agents against Mycobacterium tuberculosis by virtual screening and bioassay. J Chem Inf Model 53:343–353
Wang J, Pursell NW, Samson MES, Atoyan R, Ma AW, Selmi A, Xu W, Cai X, Voi M, Savagner P, Lai C-J (2013) Potential advantages of CUDC-101, a Multitargeted HDAC, EGFR, and HER2 inhibitor, in treating drug resistance and preventing cancer cell migration and invasion. Mol Cancer Ther 12:925–936
Xu K, Dai X-L, Huang H-C, Jiang Z-F (2011) Targeting HDACs: a promising therapy for Alzheimer’s disease. Oxid Med Cell Longev 2011:1–5
Yang Z, Wang T, Wang F, Niu T, Liu Z, Chen X, Chen L (2015) Discovery of selective histone deacetylase 6 inhibitors using the quinazoline as the cap for the treatment of cancer. J Med Chem 59:1455–1470
Yoon S, Eom GH (2016) HDAC and HDAC inhibitor: from cancer to cardiovascular diseases. Chonnam Med J 52:1
Yu C-W, Chang P-T, Hsin L-W, Chern J-W (2013) Quinazolin-4-one derivatives as selective histone deacetylase-6 inhibitors for the treatment of Alzheimer’s disease. J Med Chem 56:6775–6791
Zamora R, Grzesiok A, Weber H, Feelisch M (1995) Oxidative release of nitric oxide accounts for guanylyl cyclase stimulating, vasodilator and anti-platelet activity of Piloty’s acid: a comparison with Angeli’s salt. Biochemical J 312:333–339
Zang L, Kondengaden SM, Zhang Q, Li X, Sigalapalli DK, Kondengadan SM, Wang PG (2017) Structure based design, synthesis and activity studies of small hybrid molecules as HDAC and G9a dual inhibitors. Oncotarget 8:63187–63207
Zhang L, Han Y, Jiang Q, Wang C, Chen X, Li X, Xu W (2014) Trend of histone deacetylase inhibitors in cancer therapy: isoform selectivity or multitargeted strategy. Med Res Rev 35:63–84
Zhang Q, Li Y, Zhang B, Lu B, Li J (2017) Design, synthesis and biological evaluation of novel histone deacetylase inhibitors incorporating 4-aminoquinazolinyl systems as capping groups. Bioorg Med Chem Lett 27:4885–4888
Zhang X, Su M, Chen Y, Li J, Lu W (2013) The design and synthesis of a new class of RTK/HDAC dual-targeted inhibitors. Molecules 18:6491–6503
Zhang Y-M, Fan X, Yang S-M, Scannevin RH, Burke SL, Rhodes KJ, Jackson PF (2008a) Syntheses and in vitro evaluation of arylsulfone-based MMP inhibitors with heterocycle-derived zinc-binding groups (ZBGs). Bioorg Med Chem Lett 18:405–408
Zhang Y-M, Xiang B, Yang Sh-M, Rhodes K, Scannevin R, Jackson P, Chakravarty D, Karnachi P (2008b) Heterocyclic derived metalloprotease inhibitors. PCT International Application No. WO2008045668, 63187–63207
Zhao XZ, Smith SJ, Métifiot M, Johnson BC, Marchand C, Pommier Y, Hughes SH, Burke TR (2014) Bicyclic 1-hydroxy-2-oxo-1,2-dihydropyridine-3-carboxamide-containing HIV-1 integrase inhibitors having high antiviral potency against cells harboring raltegravir-resistant integrase mutants. J Med Chem 57:1573–1582
Zhou X, Xie X, Liu G (2013) Quinazoline-2,4(1H,3H)-diones inhibit the growth of multiple human tumor cell lines. Mol Diversity 17:197–219
Acknowledgements
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-11-2018-172 dated 03.12.18). Unique project identifier RFMEFI62418X0051.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Osipov, V.N., Khachatryan, D.S. & Balaev, A.N. Biologically active quinazoline-based hydroxamic acids. Med Chem Res 29, 831–845 (2020). https://doi.org/10.1007/s00044-020-02530-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02530-7