Abstract
Objectives
We used the results of the Global Burden of Disease 2015 study to estimate trends of HIV/AIDS burden in Eastern Mediterranean Region (EMR) countries between 1990 and 2015.
Methods
Tailored estimation methods were used to produce final estimates of mortality. Years of life lost (YLLs) were calculated by multiplying the mortality rate by population by age-specific life expectancy. Years lived with disability (YLDs) were computed as the prevalence of a sequela multiplied by its disability weight.
Results
In 2015, the rate of HIV/AIDS deaths in the EMR was 1.8 (1.4–2.5) per 100,000 population, a 43% increase from 1990 (0.3; 0.2–0.8). Consequently, the rate of YLLs due to HIV/AIDS increased from 15.3 (7.6–36.2) per 100,000 in 1990 to 81.9 (65.3–114.4) in 2015. The rate of YLDs increased from 1.3 (0.6–3.1) in 1990 to 4.4 (2.7–6.6) in 2015.
Conclusions
HIV/AIDS morbidity and mortality increased in the EMR since 1990. To reverse this trend and achieve epidemic control, EMR countries should strengthen HIV surveillance, and scale up HIV antiretroviral therapy and comprehensive prevention services.
Similar content being viewed by others
Introduction
In 2015, HIV/AIDS was the 12th-leading cause of death worldwide after being the eighth in 2005 when the epidemic peaked (Institute for Health Metrics and Evaluation (IHME) 2017). More than 1.2 million people are estimated to have died in 2015 due to HIV/AIDS despite the considerable achievements in HIV care since the late 1980s (Wang et al. 2016b). This reflects the challenges faced by public health policymakers and program managers, health professionals, and the global community in dealing with this epidemic.
The burden of the HIV/AIDS epidemic has rapidly changed since the 1990s with the introduction of HIV antiretroviral therapy (ART) and other effective interventions (UNAIDS 2015). While incidence has declined continuously since the mid-1990s, mortality continued to rise and peaked in 2005 at 1.8 million deaths worldwide (Wang et al. 2016b). Inspired by the successes of responding to AIDS, global leaders have committed to and embarked on ending the AIDS epidemic as a public health threat by 2030, without leaving anyone behind (UNAIDS 2014a). Today, there are large variations in incidence and mortality between regions and countries (Wang et al. 2016b). In the Eastern Mediterranean Region (EMR), and despite recent progress (Institute for Health Metrics and Evaluation (IHME) 2017), estimates of HIV/AIDS continue to be challenged with limitations in data availability and by insufficient epidemiological surveillance among those most at-risk of infection (Shawky et al. 2009; Mumtaz et al. 2014a). The EMR has a population of about 583 million people. Countries in the EMR vary significantly in terms of their gross domestic product, socio-demographic profiles, health indicators, and health system capacities and coverage (WHO EMRO 2017).
The EMR has several vulnerability factors for HIV (Abu-Raddad et al. 2010). The socio-cultural and socioeconomic fabric as well as the demographic structure of the region is evolving rapidly (Abu-Raddad et al. 2010). Extensive levels of migration, displacement, mobility, and conflicts are a hallmark of the region (UNAIDS RST MENA 2008). Injection drug use is also a major challenge in a region that produces most of the world’s supply of heroin and is at the crossroads of major drug trade routes (UNODC 2007).
The emerging HIV epidemics among the most at-risk populations, such as men who have sex with men (MSM) and people who inject drugs (PWID), constitute the main feature of HIV epidemiology in the EMR today within a context that criminalizes and marginalizes these populations (Simmons 2014; Mumtaz et al. 2014a). The majority of these epidemics are recent, having emerged within the last two decades (Mumtaz et al. 2014b). In addition to these documented epidemics, there is evidence suggesting hidden, undetected epidemics among the most at-risk populations in countries with still weak HIV surveillance systems (Mumtaz et al. 2014a).
Data on disability and mortality from HIV are crucial in understanding the regional response to the disease. To inform HIV policy, programming, and resource allocation about the state of the epidemic in EMR countries, we used the results of the GBD 2015 study to report the HIV/AIDS burden in these countries.
Methods
The Eastern Mediterranean Region (EMR) countries, based on the World Health Organization classification, are the Islamic Republic of Afghanistan, the Kingdom of Bahrain, Djibouti, the Arab Republic of Egypt, the Islamic Republic of Iran, the Republic of Iraq, the Hashemite Kingdom of Jordan, the State of Kuwait, Lebanon, the State of Libya, the Kingdom of Morocco, the Sultanate of Oman, the Islamic Republic of Pakistan, Palestine, the State of Qatar, the Kingdom of Saudi Arabia, the Federal Republic of Somalia, the Republic of Sudan, the Syrian Arab Republic, the Republic of Tunisia, the United Arab Emirates, and the Republic of Yemen.
A detailed methodology of HIV/AIDS mortality estimation for GBD 2015 has been published elsewhere (Wang et al. 2016b). We used all available data sources including vital registration, verbal autopsies, surveys, publications, and reports. These data sources have been published elsewhere as an appendix (Wang et al. 2016b), and are available from the Global Health Data Exchange (Institute for Health Metrics and Evaluation 2017). Briefly, the GBD estimation framework contains three sources for estimates of HIV-specific mortality: estimated HIV mortality from Spectrum (Brown et al. 2014; Stover et al. 2014); estimated excess HIV/AIDS mortality in our all-cause mortality estimation process; and spatiotemporal Gaussian process regression smoothed cause-specific HIV/AIDS mortality from vital registration (VR) systems that were adjusted for incompleteness and misclassification of causes of death (Wang et al. 2016a). Tailored estimation methods were used to produce final estimates of mortality depending on age groups and the availability and quality of data for mortality of HIV/AIDS.
Years of life lost (YLLs) were calculated by multiplying the mortality rate by population by age-specific life expectancy from the reference life table used in the GBD study. Years lived with disability (YLDs) were computed as the prevalence of a sequela multiplied by the disability weight for that sequela without age weighting or discounting. The YLDs arising from HIV/AIDS are the sum of the YLDs for each of the sequelae associated with HIV/AIDS. Disability-adjusted life years (DALYs) are computed as the sum of YLLs and YLDs. Detailed methods on YLLs, YLDs, and DALYs are published elsewhere (GBD 2015 DALYs and HALE Collaborators 2016; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators 2016; GBD 2015 Risk Factors Collaborators 2016).
We estimated incidence and prevalence from the recoded spectrum model. This model was updated with assumptions of on-ART and off-ART mortality, as well as other program data available from the UNAIDS country files. Vital registration systems and sample registration systems provide some of the most reliable sources for estimation of HIV cause-specific deaths. Later, our cohort incidence bias adjustment method was used to scale the sizes of each incidence cohort on the basis of the raw estimates of HIV mortality from spectrum, adjusted for incompleteness and cause misclassification using unadjusted incidence curves and those observed in the vital registration system (Wang et al. 2016a). More details about this method have been published previously (Wang et al. 2016b).
We also estimated risk factors following the GBD study’s comparative assessment of risk factors detailed elsewhere (Forouzanfar et al. 2015). Briefly, this method uses data for excess mortality and disability associated with risk factors, data for exposure to risks, and evidence-based assumptions on the desired counterfactual distribution of risk exposure. The attributable burden of a risk factor is estimated by multiplying DALYs from HIV/AIDS by the population attributable fraction for HIV/AIDS due to that risk factor.
We report age-standardized estimates, and 95% uncertainty intervals (UI) for each estimate—such as rates or numbers of deaths or DALYs. We estimated UIs by taking 1000 samples from the posterior distribution of each quantity and using the 25th- and 975th-ordered draws of the uncertainty distribution (Wang et al. 2016a). For 2015, we estimated the expected burden for each of the three measures (mortality, YLLs, and YLDs) as a function of each country’s Socio-demographic Index (SDI)—a composite measure based on levels of income—education, and fertility (Wang et al. 2016a). SDI was developed for GBD 2015 to provide an interpretable synthesis of overall development, as measured by lag-dependent income per capita, average educational attainment in the population over 15 years of age, and total fertility rates. In GBD 2015, SDI was computed by rescaling each component to a scale of zero to one, with zero being the lowest observed educational attainment, lowest income per capita, and highest fertility rate from 1980 to 2015, and one being the highest observed educational attainment, highest income per capita, and lowest fertility rate during that time, and then taking the geometric mean of these values for each location-year.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Mortality
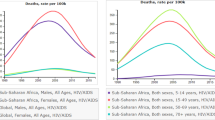
The proportion of deaths attributable to HIV/AIDS has increased steadily in the EMR since 1990 by 6.7% annually (Fig. 1).
In 2015, HIV/AIDS caused 10,558 (95% UI 8411–17,775) deaths in the EMR, a tenfold increase from 1990 (936; 470–2226). This equals an increase in age-standardized rate from 0.3 (0.2–0.8) in 1990 to 1.8 (1.4–2.5) per 100,000 population in 2015 (Table 1). HIV/AIDS mortality among males—2.4 (1.8–3.4) deaths per 100,000 population—was double that among females—1.1 (0.9–1.5) deaths per 100,000 population. It affected mostly infants and those aged 25 years or older (Fig. 2). HIV/AIDS deaths as a percentage of all deaths decreased in Kuwait, Lebanon and, Syria at an annualized rate of 3.3, 1.0, and 0.4%, respectively (Table 2). In 2015, the percent of deaths due to HIV/AIDS was highest in Djibouti, and higher than the regional average, 0.2 (0.1–0.2), in Bahrain, Oman, Libya, Lebanon, Saudi Arabia Somalia, Sudan, and UAE. It was lower than the regional average in all remaining countries (Table 2).
Years of life lost
Years of life lost to HIV/AIDS increased from 49,094 (24,960–117,290) in 1990 to 526,030 (416,745–734,351) in 2015. The rate of YLLs increased as well for the same period from 15.3 (7.6–36.2) to 81.8 (65.3–114.4) per 100,000 population (e-Table 1). E-Table 1 shows these rates for individual countries. The percent of YLLs due to HIV/AIDS decreased in Kuwait, Lebanon, Syria, and Qatar at annualized rates of 3.4, 1.4, 0.3, and 0.1%, respectively (Table 2).
Years lived with disability
HIV/AIDS accounted for 26,000 (16,440–38,839) YLDs in 2015, a sixfold increase from 3829 (1875–8539) in 1990. The rate increased from 1.3 (0.6–3.1) per 100,000 population in 1990 to 4.4 (2.7–6.6) in 2015 (e-Table 2). E-Table 2 shows these rates for individual countries. The percent of YLDs due to HIV/AIDS decreased in Lebanon, Qatar, and Yemen by annualized rates of 2.2, 1.3, and 0.9%, respectively (Table 2).
HIV/AIDS caused more YLLs than YLDs at all times (e-Fig. 1).
Disability-adjusted life years
DALYs due to HIV/AIDS increased tenfold between 1990—52,923 (26,913–124,169)—and 2015—552,030 (439,956–768,775). The rate increased from 16.6 (8.4–38.8) to 86.2 (69.2–120.6) per 100,000 population (e-Table 3). E-Table 3 shows these rates for individual countries. The percent of DALYs due to HIV/AIDS decreased in Kuwait, Lebanon, Qatar, Syria, and Yemen by annualized rates of 3.3, 1.5, 0.2, 0.2, and 0.1%, respectively (Table 2).
Incidence and prevalence
Incidence and prevalence of HIV/AIDS have increased in the EMR since 1990 from 2.9 (2.0–4.9) and 9.1 (5.1–16.4), to 5.3 (3.9–7.9) and 28.4 (22.3–39.8) per 100,000 population, respectively. The highest and lowest incidence for 2015 was observed in Djibouti and Syria, respectively: 90.9 (55.0–142.4) and 0.4 (0.2–0.5). The highest and lowest prevalence for 2015 were observed in Djibouti and Kuwait, respectively: 919.7 (714.8–1161.9) and 0.0 (0.0–0.0) per 100,000 populations. Table 3 presents estimates of incidence and prevalence of HIV/AIDS in EMR countries in 1990, 2005, and 2015.
Risk factors
Unsafe sex and drug use accounted for 74.1 and 18.8% of HIV deaths, 75.3 and 17.5% of HIV YLLs, 71.9 and 21.3% of HIV YLDs, and 75.1 and 17.7% of HIV DALYs, respectively. In Djibouti, where HIV/AIDS mortality was highest in comparison to all other EMR countries, unsafe sex and drug use contributed to 94.4 and 0.4% of deaths related to HIV/AIDS, respectively. On the other hand, in Syria, where HIV/AIDS mortality was lowest, unsafe sex and drug use contributed to 84.5 and 6.4% of deaths related to HIV/AIDS, respectively. Table 4 presents estimates of risk factors contribution to HIV/AIDS deaths, YLLs, YLDs, and DALYs.
Observed versus expected burden
Despite the increase of HIV/AIDS mortality in EMR countries over time, all, but Djibouti had lower observed deaths than expected based on SDI (Table 1). Expected deaths were within the range of uncertainty for the observed deaths in Djibouti (Table 1). Only Djibouti had higher observed YLLs and YLDs than what would have been expected for 2015 based on SDI (e-Tables 1, 2). Expected YLDs were within the range of uncertainty for the observed YLDs in Bahrain, Lebanon, Libya, Saudi Arabia, and the United Arab Emirates (e-Table 2). Expected DALYs were within the range of uncertainty for the observed DALYs in Djibouti and the United Arab Emirates (e-Table 3).
Discussion
This is the first GBD study to comprehensively examine the burden and trends of HIV/AIDS-related mortality in EMR countries from 1990 to 2015. Our estimates show a tenfold increase in HIV/AIDS mortality rates and other measures of disease burden for the EMR region with most of the HIV/AIDS burden is contributed by the three poorest countries Djibouti, Somalia, and Sudan. These results highlight the expanding nature of the epidemic in the EMR, in contrast to the other global regions (UNAIDS 2016a). They also affirm the epidemiological evidence indicating emerging HIV epidemics within the last two decades such as among MSM in nearly half of EMR countries (Mumtaz et al. 2011, 2014a) and among PWID in over a third of EMR countries (Mumtaz et al. 2014a, b), two populations that are still being criminalized in this region, making epidemic control harder to reach (Simmons 2014; Aaraj and Chrouch 2016). Despite these rapid increases, HIV disease burden in EMR remains at least tenfold lower than HIV/AIDS mortality at the global level, and at all times (Wang et al. 2016b).
These results indicate that EMR countries are not likely to fulfill the Joint United Nations Program on HIV/AIDS (UNAIDS) “90-90-90” target of diagnosing 90% of all people living with HIV/AIDS, providing ART for 90% of those diagnosed, and achieving viral suppression for 90% of those treated, all by 2020 (UNAIDS 2014b). EMR countries are also not likely to reach the fast-track target of ending AIDS by 2030 (UNAIDS 2016a). The striking gap between the expanding disease burden and global targets for reducing this burden highlights the need for EMR countries to strengthen HIV/AIDS voluntary counseling and testing among the most at-risk populations, improve HIV epidemiological surveillance, and scale up ART and comprehensive prevention services.
A major challenge in the EMR is the weak vital registration and epidemiological surveillance systems. People living with HIV are being diagnosed at a late stage of disease progression, thus their chances of accessing treatment and surviving are decreasing. Most HIV infections appear to be detected through routine screening, such as in the context of blood donation, premarital medical tests, and employment, or visa and residency applications (Hermez et al. 2010). Moreover, data on relevant HIV/AIDS indicators, such as the Global AIDS Response Progress Reporting indicators, are limited in many EMR countries, although quality integrated bio-behavioral surveillance surveys (IBBSS) of hard-to-reach populations have already proven possible in over half of EMRO countries (Abu-Raddad et al. 2010; Mumtaz et al. 2011, 2014b). Sustainability of IBBSS rounds in countries where they have been conducted, and implementing them in countries where they have not been conducted, should be a priority.
These results also affirm the evidence indicating low ART coverage in EMR and persistent challenges with the treatment cascade (World Health Organization 2017; UNAIDS 2016b). EMR has the lowest ART coverage globally at a median of 17% in 2015 (UNAIDS 2016b), and did not reach the 2015 midterm regional objective of 50% coverage under the World Health Organization’s (WHO) initiative to end EMR’s HIV treatment crisis (World Health Organization Regional Office for the Eastern Mediterranean 2014).
The effectiveness of highly active ART was manifested in 1995 and became the new standard for HIV care in 1997, making HIV a manageable disease (Carpenter et al. 1997; Palmisano and Vella 2011). Despite this progress, only three EMR countries showed a decrease in HIV/AIDS mortality. These countries can share lessons with the remaining EMR countries to help them control their epidemics. Moreover, our study showed that for most EMR countries, the increase in YLLs exceeded by far the increase in YLDs during the study period. For instance, while YLDs contributed to 4.7% of HIV/AIDS DALYs in EMR countries, they contributed to 8.4% in European countries (Institute for Health Metrics and Evaluation (IHME) 2017). This indicates that HIV survival is very low in EMR countries, affirming the weak and challenged HIV/AIDS response in this region (Abu-Raddad et al. 2013). Even if HIV/AIDS treatment is available, often it is interrupted and patients struggle to survive. Most health care providers are also not well trained to manage HIV/AIDS patients and/or understand their situations (Khosravanifard et al. 2012; Wilder 2008; Anonymous 2012; Thayer 2012; Upham and Mikkelsen 2012; Hedayati-Moghaddam et al. 2012).
Interestingly, the observed burden of HIV/AIDS was lower than expected in most EMR countries based on their SDI. On the surface, this might be sound like good news. However, the burden of HIV/AIDS has been increasing continuously in the EMR despite the decrease in the rest of the world. While SDI is known to be a strong indicator of health outcomes (Wang et al. 2016a), it is possible that the association with HIV/AIDS is modified by other cultural and social factors in the EMR. SDI only deals with socioeconomic inequalities between countries and does not account for other cultural and social norms. For instance, more of the risky behaviors for HIV, such as access to drugs and alcohol, travel, or multiplicity of concurrent relationships might be more common among higher-SDI groups in the EMR. Further, some of the EMR countries have experienced warfare and conflicts, highlighting the difference in the social determinants of HIV in conflict versus non-conflict settings, with HIV morbidity and mortality closely associated with conflicts (Betsi et al. 2006; Mowafi 2011; Wirtz et al. 2014; Robertson and Hoffman 2014; Doocy et al. 2015; Tunçalp et al. 2015; Calam 2016). Some of these include sexual violence and human rights abuses in conflict settings, interruption of treatment due to mass displacement, disruption of health systems, and resource diversions from health to support wars. However, SDI allows comparisons between countries based on similar indicators, and hence is important to use, despite its limitations.
Meanwhile, the poor management and treatment of HIV/AIDS patients is also a persistent issue. EMR policymakers need to devote adequate funds to expand HIV prevention and treatment services even if the leading causes of deaths, YLLs, and YLDs in the EMR are non-communicable, such as ischemic heart disease, diabetes, and road injuries (Mokdad et al. 2014, 2016). These services need to be expanded, particularly among the most at-risk populations. Countries need to put in place active surveillance systems to detect early infections and monitor the epidemic, in addition to delivering health care to those affected. With drug use playing a significant role in HIV transmission in this region, introducing syringe exchange programs should be considered given its proven effectiveness in preventing HIV transmission (Wilson et al. 2015).
Our study might be subjected to several limitations around the estimation of HIV/AIDS burden. These limitations have been previously described (Wang et al. 2016b). In short, our study estimates mortality with HIV/AIDS as the underlying cause of death without accounting for deaths from other non-communicable causes among people living with HIV. Additionally, data are less available for the most recent years, and our models might have missed recent progress, or lack of it, in some countries. Our estimates have not accounted directly for relevant covariates including prevalence of sexually transmitted infections or rates of ART adherence, ART treatment failure, and HIV testing (Wang et al. 2016b).
Our study showed that HIV/AIDS disease burden is increasing in the vast majority of EMR countries, in contrast to the global declining trend. Increased and coordinated efforts are needed in the region to apply lessons from countries that have succeeded in controlling their epidemic to reduce this burden, reverse its trend, and reach global stipulated targets for HIV/AIDS. More affluent EMR countries must consider ways to bring the region’s more disadvantaged countries to the same level of health. These findings highlight the need for EMR countries to strengthen HIV/AIDS voluntary counseling and testing among the most at-risk populations, improve HIV epidemiological surveillance, and scale up ART and comprehensive prevention services.
References
Aaraj E, Chrouch MJA (2016) Drug policy and harm reduction in the Middle East and North Africa: the role of civil society. Int J Drug Policy 31:168–171. doi:10.1016/j.drugpo.2016.03.002
Abu-Raddad LJ, Semini I, Riedner G et al (2010) Characterizing the HIV/AIDS epidemic in the Middle East and North Africa : time for strategic action. The World Bank, Washington, DC
Abu-Raddad L, Sgaier S, Mumtaz G (2013) HIV response in the Middle East and North Africa: an epidemic and its policy dilemmas. In: Smith R (ed) Global HIV/AIDS politics, policy and activism: persistent challenges and emerging issues. Praeger Publishers, Washington DC, pp 143–168
Anonymous (2012) Iran’s HIV/Aids sufferers struggle for survival. BBC News, December 3rd, 2012
Betsi NA, Koudou BG, Cissé G et al (2006) Effect of an armed conflict on human resources and health systems in Côte d’Ivoire: prevention of and care for people with HIV/AIDS. AIDS Care 18:356–365. doi:10.1080/09540120500200856
Brown T, Bao L, Eaton JW et al (2014) Improvements in prevalence trend fitting and incidence estimation in EPP 2013. AIDS 28(Suppl 4):S415–S425. doi:10.1097/QAD.0000000000000454
Calam R (2016) Public health implications and risks for children and families resettled after exposure to armed conflict and displacement. Scand J Public Health. doi:10.1177/1403494816675776
Carpenter CC, Fischl MA, Hammer SM et al (1997) Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA panel. JAMA 277:1962–1969
Doocy S, Lyles E, Delbiso TD et al (2015) Internal displacement and the Syrian crisis: an analysis of trends from 2011–2014. Confl Health 9:33. doi:10.1186/s13031-015-0060-7
Forouzanfar MH, Alexander L, Anderson HR et al (2015) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:2287–2323. doi:10.1016/S0140-6736(15)00128-2
GBD 2015 DALYs and HALE Collaborators (2016) Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1603–1658. doi:10.1016/S0140-6736(16)31460-X
GBD 2015 Disease and Injury Incidence and Prevalence Collaborators (2016) Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602. doi:10.1016/S0140-6736(16)31678-6
GBD 2015 Risk Factors Collaborators (2016) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1659–1724. doi:10.1016/S0140-6736(16)31679-8
Hedayati-Moghaddam MR, Marjaneh MM, Mashhadi IE (2012) Knowledge and attitudes of physicians in private practice towards HIV/AIDS in Mashhad, Iran. Int J STD AIDS 23:e11–e16. doi:10.1258/ijsa.2009.009447
Hermez J, Petrak J, Karkouri M, Riedner G (2010) A review of HIV testing and counseling policies and practices in the Eastern Mediterranean Region. AIDS 24(Suppl 2):S25–S32. doi:10.1097/01.aids.0000386730.56683.e5
Institute for Health Metrics and Evaluation (IHME) (2017) Global Burden of Disease data visualization. IHME, University of Washington, Seattle
Institute for health metrics and evaluation global health data exchange | GHDx (2017). http://ghdx.healthdata.org/. Accessed 24 Apr 2017
Khosravanifard B et al (2012) Tehran dentists’ self-reported knowledge and attitudes towards HIV/AIDS and observed willingness to treat simulated HIV-positive patients. East Mediterr Health J 18(9):928–934
Mokdad AH, Jaber S, Aziz MIA et al (2014) The state of health in the Arab world, 1990–2010: an analysis of the burden of diseases, injuries, and risk factors. Lancet 383:309–320. doi:10.1016/S0140-6736(13)62189-3
Mokdad AH, Forouzanfar MH, Daoud F et al (2016) Health in times of uncertainty in the eastern Mediterranean region, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Glob Health 4:e704–e713. doi:10.1016/S2214-109X(16)30168-1
Mowafi H (2011) Conflict, displacement and health in the Middle East. Glob Public Health 6:472–487. doi:10.1080/17441692.2011.570358
Mumtaz G, Hilmi N, McFarland W et al (2011) Are HIV epidemics among men who have sex with men emerging in the middle East and North Africa? A systematic review and data synthesis. PLoS Med 8:e1000444. doi:10.1371/journal.pmed.1000444
Mumtaz GR, Riedner G, Abu-Raddad LJ (2014a) The emerging face of the HIV epidemic in the Middle East and North Africa. Curr Opin HIV AIDS 9:183–191. doi:10.1097/COH.0000000000000038
Mumtaz GR, Weiss HA, Thomas SL et al (2014b) HIV among people who inject drugs in the Middle East and North Africa: systematic review and data synthesis. PLoS Med 11:e1001663. doi:10.1371/journal.pmed.1001663
Palmisano L, Vella S (2011) A brief history of antiretroviral therapy of HIV infection: success and challenges. Ann Ist Super Sanita 47:44–48. doi:10.4415/ANN_11_01_10
Robertson CL, Hoffman SJ (2014) Conflict and forced displacement: human migration, human rights, and the science of health. Nurs Res 63:307–308
Shawky S, Soliman C, Kassak KM et al (2009) HIV surveillance and epidemic profile in the Middle East and North Africa. J Acquir Immune Defic Syndr 51:S83–S95. doi:10.1097/QAI.0b013e3181aafd3f
Simmons H (2014) Dying for love: homosexuality in the Middle East. Human rights and human welfare (Issue on Human Rights in the Middle East and North Africa), pp 160–172
Stover J, Andreev K, Slaymaker E et al (2014) Updates to the spectrum model to estimate key HIV indicators for adults and children. AIDS 28:S427–S434. doi:10.1097/QAD.0000000000000483
Thayer T (2012) AIDS in Iran-life or death. AIDS Response Effort Inc, Winchester
Tunçalp Ö, Fall IS, Phillips SJ et al (2015) Conflict, displacement and sexual and reproductive health services in Mali: analysis of 2013 health resources availability mapping system (HeRAMS) survey. Confl Health 9:28. doi:10.1186/s13031-015-0051-8
UNAIDS (2014a) Fast-track, ending the AIDS epidemic by 2030. UNAIDS, Geneva
UNAIDS (2014b) 90-90-90 An ambitious treatment target to help the AIDS epidemic. Joint United Nations Programme on HIV/AIDS, Geneva
UNAIDS (2015) How AIDS changed everything—MDG6: 15 years, 15 lessons of hope from the AIDS response. UN, New York City
UNAIDS (2016a) Prevention gap report. Joint United Nations Programme on HIV/AIDS, Geneva
UNAIDS (2016b) Global AIDS update 2016. Joint United Nations Programme on HIV/AIDS, Geneva
UNAIDS RST MENA (2008) Notes on AIDS in the Middle East and North Africa. Cairo, Egypt
UNODC (2007) United Nations office on drugs and crime
Upham S, Mikkelsen L (2012) Advocacy for strengthening civil registration and vital statistics. Pac Health Dialog 18:41–52
Wang H, Naghavi M, Allen C et al (2016a) Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1459–1544. doi:10.1016/S0140-6736(16)31012-1
Wang H, Wolock TM, Carter A et al (2016b) Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV 3:e361–e387. doi:10.1016/S2352-3018(16)30087-X
WHO EMRO (2017) About us. http://www.emro.who.int/entity/about-us/index.html. Accessed 24 Apr 2017
Wilder T (2008) In Lebanon, fighting for a better HIV-positive future. An interview with Margarita, an HIV-positive Lebanese woman, August 1, 2008. http://www.thebody.com/content/art47923.html
Wilson DP, Donald B, Shattock AJ et al (2015) The cost-effectiveness of harm reduction. Int J Drug Policy 26(Suppl 1):S5–S11. doi:10.1016/j.drugpo.2014.11.007
Wirtz AL, Pham K, Glass N et al (2014) Gender-based violence in conflict and displacement: qualitative findings from displaced women in Colombia. Confl Health 8:10. doi:10.1186/1752-1505-8-10
World Health Organization Regional database on HIV/AIDS (2017)
World Health Organization Regional Office for the Eastern Mediterranean (2014) From HIV testing to lifelong care and treatment—access to the continuum of HIV care and treatment in the Eastern Mediterranean Region, Progress Report 2014
GBD 2015 Eastern Mediterranean Region HIV/AIDS Collaborators:
Ali H. Mokdad, PhD (corresponding author), Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Charbel El Bcheraoui PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Haidong Wang, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Raghid Charara, MD, American University of Beirut, Beirut, Lebanon. Ibrahim Khalil, MD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Maziar Moradi-Lakeh, MD, Department of Community Medicine, Preventive Medicine and Public Health Research Center, Gastrointestinal and Liver Disease Research Center (GILDRC), Iran University of Medical Sciences, Tehran, Iran. Ashkan Afshin, MD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Michael Collison, BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Farah Daoud, BA/BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Adrienne Chew, ND, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Kristopher J. Krohn, BA, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Austin Carter, BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Kyle J. Foreman, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States, Imperial College London, London, UK. Fei He, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Nicholas J. Kassebaum, MD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States; Department of Anesthesiology & Pain Medicine, Seattle Children’s Hospital, Seattle, Washington, United States. Michael Kutz, BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Mojde Mirarefin, MPH, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States; Hunger Action Los Angeles, Los Angeles, CA, United States. Grant Nguyen, MPH, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Naris Silpakit, BS, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Amber Sligar, MPH, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Amanuel Alemu Abajobir, MPH, School of Public Health, University of Queensland, Brisbane, QLD, Australia. Kalkidan Hassen Abate, MS, Jimma University, Jimma, Ethiopia. Kaja M. Abbas, PhD, Virginia Tech, Blacksburg, VA, United States. Foad Abd-Allah, MD, Department of Neurology, Cairo University, Cairo, Egypt. Semaw Ferede Abera, MSc, School of Public Health, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; Food Security and Institute for Biological Chemistry and Nutrition, University of Hohenheim, Stuttgart, Germany. Kelemework Adane, MS, Department of Medical Microbiology and Immunology, College of Health Sciences, Mekelle University, Mekelle, Ethiopia. Arnav Agarwal, BHSc, University of Toronto, Toronto, Ontario, Canada; McMaster University, Hamilton, Ontario, Canada. Aliasghar Ahmad Kiadaliri, PhD, Department of Clinical Sciences Lund, Orthopedics, Clinical Epidemiology Unit, Lund University, Lund, Sweden. Alireza Ahmadi, PhD, Kermanshah University of Medical Sciences, Kermanshah, Iran. Muktar Beshir Ahmed, MPH, College of Health Sciences, Department of Epidemiology, ICT and e-Learning Coordinator, Jimma University, Jimma, Ethiopia. Faris Hasan Al Lami, PhD, Baghdad College of Medicine, Baghdad, Iraq. Khurshid Alam, PhD, Murdoch Childrens Research Institute, The University of Melbourne, Parkville, Victoria, Australia; The University of Melbourne, Melbourne, VIC, Australia; The University of Sydney, Sydney, NSW, Australia. Deena Alasfoor, MSc, Ministry of Health, Al Khuwair, Oman. Reza Alizadeh-Navaei, PhD, Gastrointestinal Cancer Research Center, Mazandaran University of Medical Sciences, Sari, Iran. Fatma Al-Maskari, PhD, College of Medicine & Health Sciences, United Arab Emirates University, Al-Ain City, United Arab Emirates. Rajaa Al-Raddadi, PhD, Joint Program of Family and Community Medicine, Jeddah, Saudi Arabia. Khalid A. Altirkawi, MD, King Saud University, Riyadh, Saudi Arabia. Nelson Alvis-Guzman, PhD, Universidad de Cartagena, Cartagena de Indias, Colombia. Walid Ammar, PhD, Ministry of Public Health, Beirut, Lebanon. Nahla Anber, PhD, Mansoura University, Mansoura, Egypt. Carl Abelardo T. Antonio, MD, Department of Health Policy and Administration, College of Public Health, University of the Philippines Manila, Manila, Philippines. Palwasha Anwari, MD, Self-employed, Kabul, Afghanistan. Hamid Asayesh, PhD, Department of Medical Emergency, School of Paramedic, Qom University of Medical Sciences, Qom, Iran. Rana Jawad Asghar, MD, South Asian Public Health Forum, Islamabad, Pakistan. Tesfay Mehari Atey, MS, Mekelle University, Mekelle, Ethiopia. Euripide Frinel G. Arthur Avokpaho, MD, Institut de Recherche Clinique du Bénin (IRCB), Cotonou, Benin; Laboratoire d'Etudes et de Recherche-Action en Santé (LERAS Afrique), Parakou, Benin. Tadesse Awoke Ayele, MS, University of Gondar, Gondar, Ethiopia. Peter Azzopardi, PhD, Burnet Institute, Melbourne, Victoria, Australia; Murdoch Childrens Research Institute, Melbourne, VIC, Australia; Department of Paediatrics, The University of Melbourne, Melbourne, VIC, Australia; Wardliparingga Aboriginal Research Unit, South Australian Health and Medical Research Institute, Adelaide, South Australia, Australia. Umar Bacha PhD, School of Health Sciences, University of Management and Technology, Lahore, Pakistan. Aleksandra Barac, PhD, Faculty of Medicine, University of Belgrade, Belgrade, Serbia. Till Bärnighausen, MD, Department of Global Health and Population, Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA, United States; Africa Health Research Institute, Mtubatuba, South Africa; Institute of Public Health, Heidelberg University, Heidelberg, Germany. Shahrzad Bazargan-Hejazi, PhD, College of Medicine, Charles R. Drew University of Medicine and Science, Los Angeles, CA, United States; David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA, United States. Neeraj Bedi, MD, College of Public Health and Tropical Medicine, Jazan, Saudi Arabia. Isabela M. Bensenor, PhD, University of São Paulo, São Paulo, Brazil. Adugnaw Berhane PhD, College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia. Pascal Obong Bessong, PhD, University of Venda, Thohoyandou, South Africa. Addisu Shunu Beyene, MPH, College of Health and Medical Science, Haramaya University, Harar, Ethiopia. Zulfiqar A. Bhutta, PhD, Centre of Excellence in Women and Child Health, Aga Khan University, Karachi, Pakistan; Centre for Global Child Health, The Hospital for Sick Children, Toronto, ON, Canada. Charles Birungi, MS, University College London, London, UK. Zahid A. Butt, PhD, Al Shifa Trust Eye Hospital, Rawalpindi, Pakistan. Lucero Cahuana-Hurtado, PhD, National Institute of Public Health, Cuernavaca, Mexico. Hadi Danawi, PhD, Walden University, Minneapolis, Minnesota, United States. José das Neves, PhD, I3S-Instituto de Investigação e Inovação em Saúde, University of Porto, Porto, Portugal; INEB-Instituto de Engenharia Biomédica, University of Porto, Porto, Portugal. Kebede Deribe, MPH, Brighton and Sussex Medical School, Brighton, UK, School of Public Health, Addis Ababa University, Addis Ababa, Ethiopia. Amare Deribew, PhD, Nuffield Department of Medicine, University of Oxford, Oxford, UK; KEMRI-Wellcome Trust Research Programme, Kilifi, Kenya. Don C. Des Jarlais, PhD, Mount Sinai Beth Israel, New York, United States; Icahn School of Medicine at Mount Sinai, New York City, New York, United States. Samath D. Dharmaratne, MD, Department of Community Medicine, Faculty of Medicine, University of Peradeniya, Peradeniya, Sri Lanka. Shirin Djalalinia, PhD, Undersecretary for Research & Technology, Ministry of Health & Medical Education, Tehran, Iran. Kerrie E. Doyle, PhD, RMIT University, Bundoora, VIC, Australia; Australian National University, Canberra, ACT, Australia. Aman Yesuf Endries, MPH, Arba Minch University, Arba Minch, Ethiopia. Babak Eshrati, PhD, Ministry of Health and Medical Education, Tehran, Iran; Arak University of Medical Sciences, Arak, Iran. Emerito Jose Aquino Faraon, MD, College of Public Health, University of the Philippines Manila, Manila, Philippines; Department of Health, Manila, Philippines. Maryam S. Farvid, PhD, Department of Nutrition, Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA, United States; Harvard/MGH Center on Genomics, Vulnerable Populations, and Health Disparities, Mongan Institute for Health Policy, Massachusetts General Hospital, Boston, MA, United States. Seyed-Mohammad Fereshtehnejad, PhD, Department of Neurobiology, Care Sciences and Society (NVS), Karolinska Institutet, Stockholm, Sweden. Tesfaye Regassa Feyissa, MPH, Wollega University, Nekemte, Ethiopia. Florian Fischer, PhD, School of Public Health, Bielefeld University, Bielefeld, Germany. Alberto L. Garcia-Basteiro, MSc, Manhiça Health Research Center, Manhiça Maputo Mozambique; Barcelona Institute for Global Health, Barcelona, Spain. Tsegaye Tewelde Gebrehiwot, MPH, Jimma University, Jimma, Ethiopia. Hailay Abrha Gesesew, MPH, Jimma University, Jimma, Ethiopia. Melkamu Dedefo Gishu, MS, Haramaya University, Dire Dawa, Ethiopia; Kersa Health and Demographic Surveillance System, Harar, Ethiopia. Elizabeth Glaser, PhD, Heller School for Social Policy and Management, Brandeis University, Waltham, MA, United States. Philimon N. Gona, PhD, University of Massachusetts Boston, Boston, Massachusetts, United States. Harish Chander Gugnani, PhD, Departments of Microbiology and Epidemiology & Biostatistics, Saint James School of Medicine, The Quarter, Anguilla. Rahul Gupta, MD, West Virginia Bureau for Public Health, Charleston, West Virginia, United States. Hassan Haghparast Bidgoli, PhD, University College London, London, UK. Gessessew Bugssa Hailu, MSc, Mekelle University, Mekelle, Ethiopia; Kilte Awlaelo Health and Demographic Surveillance System, Mekelle, Ethiopia. Randah Ribhi Hamadeh, DPhil, Arabian Gulf University, Manama, Bahrain. Mitiku Teshome Hambisa, MPH, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia. Samer Hamidi, DrPH, Hamdan Bin Mohammed Smart University, Dubai, United Arab Emirates. Hilda L. Harb, MPH, Ministry of Public Health, Beirut, Lebanon. Habtamu Abera Hareri, MS, Addis Ababa University, Addis Ababa, Ethiopia. Nobuyuki Horita, MD, Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa, Japan. Abdullatif Husseini, PhD, Institute of Community and Public Health, Birzeit University, Birzeit, Palestine. Ahmed Ibrahim, MD, Alshaab Teaching Hospital, Khartoum, Sudan. Spencer Lewis James, MD, Denver Health/University of Colorado, Denver, CO, United States. Jost B. Jonas, MD, Department of Ophthalmology, Medical Faculty Mannheim, Ruprecht-Karls-University Heidelberg, Mannheim, Germany. Amir Kasaeian, PhD, Hematology-Oncology and Stem Cell Transplantation Research Center, Tehran University of Medical Sciences, Tehran, Iran; Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran. Nigussie Assefa Kassaw, MPH, Addis Ababa University, Addis Ababa, Ethiopia. Yousef Saleh Khader, ScD, Department of Community Medicine, Public Health and Family Medicine, Jordan University of Science and Technology, Irbid, Jordan. Ejaz Ahmad Khan, MD, Health Services Academy, Islamabad, Punjab, Pakistan. Gulfaraz Khan, PhD, Department of Microbiology and Immunology, College of Medicine & Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates. Abdullah Tawfih Abdullah Khoja, MD, Mohammed Ibn Saudi University, Riyadh, Saudi Arabia. Jagdish Khubchandani, PhD, Department of Nutrition and Health Science, Ball State University, Muncie, Indiana, United States. Yun Jin Kim, PhD, Faculty of Chinese Medicine, Southern University College, Skudai, Malaysia. Ai Koyanagi, MD, Research and Development Unit, Parc Sanitari Sant Joan de Deu (CIBERSAM), Barcelona, Spain. Barthelemy Kuate Defo, PhD, Department of Social and Preventive Medicine, School of Public Health, University of Montreal, Montreal, Quebec, Canada; Department of Demography and Public Health Research Institute, University of Montreal, Montreal, Canada. Heidi J. Larson, PhD, Department of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK; Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Asma Abdul Latif, PhD, Department of Zoology, Lahore College for Women University, Lahore, Pakistan. Cheru Tesema Leshargie, MPH, Debre Markos University, Debre Markos, Ethiopia. Raimundas Lunevicius, PhD, Aintree University Hospital National Health Service Foundation Trust, Liverpool, UK; School of Medicine, University of Liverpool, Liverpool, UK. Mohammed Magdy Abd El Razek, MBBCH, Aswan University Hospital, Aswan Faculty of Medicine, Aswan, Egypt. Reza Majdzadeh, PhD, Knowledge Utilization Research Center and Community Based Participatory Research Center, Tehran University of Medical Sciences, Tehran, Iran. Azeem Majeed, MD, Department of Primary Care & Public Health, Imperial College London, London, England, UK. Reza Malekzadeh, MD, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Tsegahun Manyazewal, PhD, Ethiopian Public Health Association, Addis Ababa, Ethiopia. Desalegn Markos, MS, Madda Walabu University, Robe, Ethiopia. Habibolah Masoudi Farid, MD, State Welfare Organisation, Tehran, Iran. Alem Mehari, MD, College of Medicine, Howard University, Washington, DC, United States. Alemayehu B. Mekonnen, MS, University of Gondar, Gondar, Ethiopia; The University of Sydney, Sydney, NSW, Australia. Peter Memiah, PhD, University of West Florida, Pensacola, FL, United States. Ziad A. Memish, MD, Saudi Ministry of Health, Riyadh, Saudi Arabia; College of Medicine, Alfaisal University, Riyadh, Saudi Arabia. Walter Mendoza, MD, United Nations Population Fund, Lima, Peru. Melkamu Merid Mengesha, MPH, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia. Desalegn Tadese Mengistu, MS, College of Health Sciences, Mekelle University, Mekelle, Ethiopia. Haftay Berhane Mezgebe, MS, Mekelle University, Mekelle, Ethiopia. Francis Apolinary Mhimbira, MS, Ifakara Health Institute, Bagamoyo, Tanzania. Ted R. Miller, PhD, Pacific Institute for Research & Evaluation, Calverton, MD, United States; Centre for Population Health, Curtin University, Perth, WA, Australia. Ami R. Moore, PhD, University of North Texas, Denton, Texas, United States. Ghina R. Mumtaz, PhD, Infectious Disease Epidemiology Group, Weill Cornell Meicine-Qatar, Doha, Qatar. Gopalakrishnan Natarajan, DM, Madras Medical College, Chennai, India. Joel Negin, PhD, The University of Sydney, Sydney, New South Wales, Australia. Carla Makhlouf Obermeyer, DSc, Center for Research on Population and Health, Faculty of Health Sciences, American University of Beirut, Beirut, Lebanon. Felix Akpojene Ogbo, MPH, Centre for Health Research, Western Sydney University, Sydney, New South Wales, Australia. In-Hwan Oh, PhD, Department of Preventive Medicine, School of Medicine, Kyung Hee University, Seoul, South Korea. Erika Ota, PhD, St. Luke's International University, Tokyo, Japan. David M. Pereira, PhD, REQUIMTE/LAQV, Laboratório de Farmacognosia, Departamento de Química, Faculdade de Farmácia, Universidade do Porto, Porto, Portugal. Farshad Pourmalek, PhD, University of British Columbia, Vancouver, British Columbia, Canada. Mostafa Qorbani, PhD, Non-communicable Diseases Research Center, Alborz University of Medical Sciences, Karaj, Iran. Amir Radfar, MD, A T Still University, Kirksville, MO, United States. Anwar Rafay, MS, Contech International Health Consultants, Lahore, Pakistan; Contech School of Public Health, Lahore, Pakistan. Vafa Rahimi-Movaghar, MD, Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences, Tehran, Iran. Rajesh Kumar Rai, MPH, Society for Health and Demographic Surveillance, Suri, India. Usha Ram, PhD, International Institute for Population Sciences, Mumbai, India. David Laith Rawaf, MD, WHO Collaborating Centre, Imperial College London, London, UK; North Hampshire Hospitals, Basingstroke, UK; University College London Hospitals, London, UK. Salman Rawaf, MD, Imperial College London, London, UK. Andre M. N. Renzaho, PhD, Western Sydney University, Penrith, NSW, Australia. Satar Rezaei, PhD, School of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran. Mohammad Sadegh Rezai, MD, Mazandaran University of Medical Sciences, Sari, Iran. Hirbo Shore Roba, MPH, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia. Gholamreza Roshandel, PhD, Golestan Research Center of Gastroenterology and Hepatology, Golestan University of Medical Sciences, Gorgan, Iran; Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Mahdi Safdarian, MD, Sina Trauma & Surgery Research Center, Tehran University of Medical Sciences, Tehran, Iran. Saeid Safiri, PhD, Managerial Epidemiology Research Center, Department of Public Health, School of Nursing and Midwifery, Maragheh University of Medical Sciences, Maragheh, Iran. Mohammad Ali Sahraian, MD, MS Research Center, Neuroscience Institute, Tehran University of Medical Sciences, Tehran, Iran. Payman Salamati, MD, Sina Trauma and Surgery Research Center, Tehran University of Medical Sciences, Tehran, Iran. Abdallah M. Samy, PhD, Ain Shams University, Cairo, Egypt, United States. Benn Sartorius, PhD, Public Health Medicine, School of Nursing and Public Health, University of KwaZulu-Natal, Durban, South Africa; UKZN Gastrointestinal Cancer Research Centre, South African Medical Research Council (SAMRC), Durban, South Africa. Sadaf G. Sepanlou, PhD, Digestive Diseases Research Institute, Tehran University of Medical Sciences, Tehran, Iran. Masood Ali Shaikh, MD, Independent Consultant, Karachi, Pakistan. Morteza Shamsizadeh, MPH, Department of Medical Surgical Nursing, School of Nursing and Midwifery, Hamadan University of Medical Sciences, Hamadan, Iran. Ephrem Lejore Sibamo Sibamo, MPH, Wolaita Sodo University, Wolaita Sodo, Ethiopia. Jasvinder A. Singh, MD, University of Alabama at Birmingham and Birmingham Veterans Affairs Medical Center, Birmingham, Alabama, United States. Badr H. A. Sobaih, MD, King Saud University, Riyadh, Saudi Arabia. Sergey Soshnikov, PhD, Federal Research Institute for Health Organization and Informatics, Ministry of Health of the Russian Federation, Moscow, Russia. Muawiyyah Babale Sufiyan, MBA, Ahmadu Bello University, Zaria, Nigeria. Bryan L. Sykes, PhD, Departments of Criminology, Law & Society, Sociology, and Public Health, University of California, Irvine, Irvine, CA, United States. Nuno Taveira, PhD, Instituto Superior de Ciências da Saúde Egas Moniz, Almada, Portugal; Faulty of Pharmacy, Universidade de Lisboa, Lisboa, Portugal. Teketo Kassaw Tegegne, MPH, Debre Markos University, Debre Markos, Ethiopia. Arash Tehrani-Banihashemi, PhD, Preventive Medicine and Public Health Research Center, Iran University of Medical Sciences, Tehran, Iran. Tesfalidet Tekelab, MS, Wollega University, Nekemte, Ethiopia; University of Newcastle, Newcastle, New South Wales, Australia. Girma Temam Shifa, MPH, Arba Minch University, Arba Minch, Ethiopia; Addis Ababa University, Addis Ababa, Ethiopia. Mohamad-Hani Temsah, MD, King Saud University, Riyadh, Saudi Arabia. Belay Tesssema, PhD, University of Gondar, Gondar, Ethiopia. Roman Topor-Madry, PhD, Institute of Public Health, Faculty of Health Sciences, Jagiellonian University Medical College, Kraków, Poland; Faculty of Health Sciences, Wroclaw Medical University, Wroclaw, Poland. Kingsley Nnanna Ukwaja, MD, Department of Internal Medicine, Federal Teaching Hospital, Abakaliki, Nigeria. Olalekan A. Uthman, PhD, Warwick Medical School, University of Warwick, Coventry, UK. Stein Emil Vollset, DrPH, Center for Disease Burden, Norwegian Institute of Public Health, Bergen, Norway; Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Fiseha Wadilo, MS, Wolaita Sodo University, Wolaita Sodo, Ethiopia. Tolassa Wakayo, MS, Jimma University, Jimma, Ethiopia. Minyahil Alebachew Woldu, MS, Addis Ababa University, Addis Ababa, Ethiopia. Abdulhalik Workicho, MPH, Jimma University, Jimma, Ethiopia; Ghent University, Ghent, Belgium. Shimelash Bitew Workie, MPH, Wolaita Sodo University, Wolaita Sodo, Ethiopia. Mohsen Yaghoubi, MSc, School of Public Health, University of Saskatchewan, Saskatoon, Saskatchewan, Canada. Ayalnesh Zemene Yalew, MS, Mekelle University, Mekelle, Ethiopia. Hassen Hamid Yimam, MPH, Mizan Tepi University, Mizan Teferi, Ethiopia. Naohiro Yonemoto, MPH, Department of Biostatistics, School of Public Health, Kyoto University, Kyoto, Japan. Seok-Jun Yoon, PhD, Department of Preventive Medicine, College of Medicine, Korea University, Seoul, South Korea. Marcel Yotebieng, PhD, The Ohio State University, Columbus, Ohio, United States. Mustafa Z. Younis, DrPH, Jackson State University, Jackson, MS, United States. Maysaa El Sayed Zaki, PhD, Faculty of Medicine, Mansoura University, Mansoura, Egypt. Aisha O. Jumaan, PhD, Independent Consultant, Seattle, Washington, United States. Theo Vos, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Simon I. Hay, DSc, Oxford Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, University of Oxford, Oxford, UK; Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Mohsen Naghavi, PhD, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States. Christopher J. L. Murray, DPhil, Institute for Health Metrics and Evaluation, University of Washington, Seattle, Washington, United States.
Author information
Authors and Affiliations
Consortia
Ethics declarations
Ethical approval
This manuscript reflects original work that has not previously been published in whole or in part and is not under consideration elsewhere. All authors have read the manuscript and have agreed that the work is ready for submission and accept responsibility for its contents. The authors of this paper have complied with all ethical standards and do not have any conflicts of interest to disclose at the time of submission. The funding source played no role in the design of the study, the analysis and interpretation of data, and the writing of the paper. The study did not involve human participants and/or animals; therefore, no informed consent was needed.
Funding
This research was funded by the Bill & Melinda Gates Foundation.
Conflict of interest
The authors declare that they have no conflicts of interest at this time.
Additional information
This article is part of the supplement “The state of health in the Eastern Mediterranean Region, 1990–2015”.
The members of GBD (Global Burden of Disease) 2015 Eastern Mediterranean Region HIV/AIDS Collaborators are listed at the end of the article. Ali H. Mokdad, on behalf of GBD 2015 Eastern Mediterranean Region HIV/AIDS Collaborators, is the corresponding author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
GBD 2015 Eastern Mediterranean Region HIV/AIDS Collaborators. Trends in HIV/AIDS morbidity and mortality in Eastern Mediterranean countries, 1990–2015: findings from the Global Burden of Disease 2015 study. Int J Public Health 63 (Suppl 1), 123–136 (2018). https://doi.org/10.1007/s00038-017-1023-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-017-1023-0