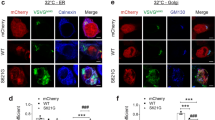
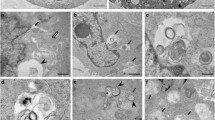
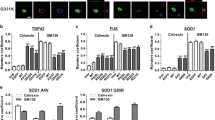
Abstract
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) are fatal neurodegenerative diseases that are related genetically and pathologically. Mutations in the UBQLN2 gene, encoding the ubiquitin-like protein ubiquilin2, are associated with familial ALS/FTD, but the pathophysiological mechanisms remain unclear. Here, we demonstrate that ALS/FTD UBQLN2 mutants P497H and P506T inhibit protein transport from the endoplasmic reticulum (ER) to the Golgi apparatus in neuronal cells. In addition, we observed that Sec31-positive ER exit sites are clustered in UBQLN2T487I patient spinal cord tissues. Both the ER–Golgi intermediate (ERGIC) compartment and the Golgi become disorganised and fragmented. This activates ER stress and inhibits ER-associated degradation. Hence, this study highlights perturbations in secretory protein trafficking and ER homeostasis as pathogenic mechanisms associated with ALS/FTD-associated forms of UBQLN2.






Similar content being viewed by others
References
Cleveland DW, Rothstein JD (2001) From charcot to lou gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2:806–819. https://doi.org/10.1038/35097565
Rothstein JD (2009) Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol 65:S3–S9
Wijesekera LC, Leigh PN (2009) Amyotrophic lateral sclerosis. Orphanet J Rare Dis 4:3
Yamashita S, Ando Y (2015) Genotype-phenotype relationship in hereditary amyotrophic lateral sclerosis. Transl Neurodegener. https://doi.org/10.1186/s40035-015-0036-y
Taylor JP, Brown RH, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539:197–206. https://doi.org/10.1038/nature20413
Deng HX, Chen W, Hong ST et al (2011) Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature 477:211–215
Fahed AC, McDonough B, Gouvion CM et al (2014) UBQLN2 mutation causing heterogeneous X-linked dominant neurodegeneration. Ann Neurol 75:793–798. https://doi.org/10.1002/ana.24164
Gellera C, Tiloca C, Bo RD et al (2012) Ubiquilin 2 mutations in Italian patients with amyotrophic lateral sclerosis and frontotemporal dementia. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2012-303433
Synofzik M, Maetzler W, Grehl T et al (2012) Screening in ALS and FTD patients reveals 3 novel UBQLN2 mutations outside the PXX domain and a pure FTD phenotype. Neurobiol Aging 33:2949.e13–2949.e17. https://doi.org/10.1016/j.neurobiolaging.2012.07.002
Williams KL, Warraich ST, Yang S et al (2012) UBQLN2/ubiquilin 2 mutation and pathology in familial amyotrophic lateral sclerosis. Neurobiol Aging 33:2527.e3–2527.e10. https://doi.org/10.1016/j.neurobiolaging.2012.05.008
Ling S-C, Polymenidou M, Cleveland DW (2013) Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79:416–438. https://doi.org/10.1016/j.neuron.2013.07.033
Lin G, Mao D, Bellen HJ (2017) Amyotrophic lateral sclerosis pathogenesis converges on defects in protein homeostasis associated with TDP-43 mislocalization and proteasome-mediated degradation overload. Curr Top Dev Biol 121:111–171. https://doi.org/10.1016/bs.ctdb.2016.07.004
Shahheydari H, Ragagnin A, Walker AK et al (2017) Protein quality control and the amyotrophic lateral sclerosis/frontotemporal dementia continuum. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2017.00119
Parakh S, Perri ER, Jagaraj CJ et al (2018) Rab-dependent cellular trafficking and amyotrophic lateral sclerosis. Crit Rev Biochem Mol Biol 53:623–651. https://doi.org/10.1080/10409238.2018.1553926
Blokhuis AM, Groen EJN, Koppers M et al (2013) Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol 125:777–794. https://doi.org/10.1007/s00401-013-1125-6
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):S10–S17. https://doi.org/10.1038/nm1066
Picher-Martel V, Dutta K, Phaneuf D et al (2015) Ubiquilin-2 drives NF-κB activity and cytosolic TDP-43 aggregation in neuronal cells. Mol Brain 8:71. https://doi.org/10.1186/s13041-015-0162-6
Ceballos-Diaz C, Rosario AM, Park H-J et al (2015) Viral expression of ALS-linked ubiquilin-2 mutants causes inclusion pathology and behavioral deficits in mice. Mol Neurodegener 10:25. https://doi.org/10.1186/s13024-015-0026-7
Alexander EJ, Ghanbari Niaki A, Zhang T et al (2018) Ubiquilin 2 modulates ALS/FTD-linked FUS-RNA complex dynamics and stress granule formation. Proc Natl Acad Sci USA 115:E11485–E11494. https://doi.org/10.1073/pnas.1811997115
Ghaemmaghami S, Huh W-K, Bower K et al (2003) Global analysis of protein expression in yeast. Nature 425:737–741. https://doi.org/10.1038/nature02046
Hauri HP, Kappeler F, Andersson H, Appenzeller C (2000) ERGIC-53 and traffic in the secretory pathway. J Cell Sci 113(Pt 4):587–596
Routledge KE, Gupta V, Balch WE (2010) Emergent properties of proteostasis-COPII coupled systems in human health and disease. Mol Membr Biol 27:385–397. https://doi.org/10.3109/09687688.2010.524894
Schröder M (2007) Endoplasmic reticulum stress responses. Cell Mol Life Sci 65:862–894. https://doi.org/10.1007/s00018-007-7383-5
Stephens DJ, Lin-Marq N, Pagano A et al (2000) COPI-coated ER-to-Golgi transport complexes segregate from COPII in close proximity to ER exit sites. J Cell Sci 113(Pt 12):2177–2185
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
McCaffrey K, Braakman I (2016) Protein quality control at the endoplasmic reticulum. Essays Biochem 60:227–235. https://doi.org/10.1042/EBC20160003
Hirsch C, Gauss R, Horn SC et al (2009) The ubiquitylation machinery of the endoplasmic reticulum. Nature 458:453–460. https://doi.org/10.1038/nature07962
Hitomi J, Katayama T, Eguchi Y et al (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J Cell Biol 165:347–356. https://doi.org/10.1083/jcb.200310015
Shore GC, Papa FR, Oakes SA (2011) Signaling cell death from the endoplasmic reticulum stress response. Curr Opin Cell Biol 23:143–149. https://doi.org/10.1016/j.ceb.2010.11.003
Urra H, Dufey E, Lisbona F et al (2013) When ER stress reaches a dead end. Biochimica et Biophysica Acta (BBA) Mol Cell Res 1833:3507–3517. https://doi.org/10.1016/j.bbamcr.2013.07.024
Ilieva EV, Ayala V, Jové M et al (2007) Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain 130:3111–3123. https://doi.org/10.1093/brain/awm190
Atkin JD, Farg MA, Walker AK et al (2008) Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis 30:400–407
Saxena S, Cabuy E, Caroni P (2009) A role for motoneuron subtype–selective ER stress in disease manifestations of FALS mice. Nat Neurosci 12:627–636. https://doi.org/10.1038/nn.2297
Saxena S, Roselli F, Singh K et al (2013) Neuroprotection through excitability and mTOR required in ALS motoneurons to delay disease and extend survival. Neuron 80:80–96. https://doi.org/10.1016/j.neuron.2013.07.027
Farg MA, Soo KY, Walker AK et al (2012) Mutant FUS induces endoplasmic reticulum stress in amyotrophic lateral sclerosis and interacts with protein disulfide-isomerase. Neurobiol Aging 33:2855–2868. https://doi.org/10.1016/j.neurobiolaging.2012.02.009
Soo KY, Halloran M, Sundaramoorthy V et al (2015) Rab1-dependent ER-Golgi transport dysfunction is a common pathogenic mechanism in SOD1, TDP-43 and FUS-associated ALS. Acta Neuropathol. https://doi.org/10.1007/s00401-015-1468-2
Sundaramoorthy V, Walker AK, Yerbury J et al (2013) Extracellular wildtype and mutant SOD1 induces ER-Golgi pathology characteristic of amyotrophic lateral sclerosis in neuronal cells. Cell Mol Life Sci 70:4181–4195. https://doi.org/10.1007/s00018-013-1385-2
Sundaramoorthy V, Walker AK, Tan V et al (2015) Defects in optineurin and myosin VI mediated cellular trafficking in amyotrophic lateral sclerosis. Hum Mol Genet. https://doi.org/10.1093/hmg/ddv126
Walker AK, Soo KY, Sundaramoorthy V et al (2013) ALS-associated TDP-43 induces endoplasmic reticulum stress, which drives cytoplasmic TDP-43 accumulation and stress granule formation. PLoS One. https://doi.org/10.1371/journal.pone.0081170
Chang HJ, Jesch SA, Gaspar ML, Henry SA (2004) Role of the unfolded protein response pathway in secretory stress and regulation of INO1 expression in Saccharomyces cerevisiae. Genetics 168:1899–1913. https://doi.org/10.1534/genetics.104.032961
Leber JH, Bernales S, Walter P (2004) IRE1-independent gain control of the unfolded protein response. PLoS Biol 2:e235. https://doi.org/10.1371/journal.pbio.0020235
Higashio H, Kohno K (2002) A genetic link between the unfolded protein response and vesicle formation from the endoplasmic reticulum. Biochem Biophys Res Commun 296:568–574
Sato M, Sato K, Nakano A (2002) Evidence for the intimate relationship between vesicle budding from the ER and the unfolded protein response. Biochem Biophys Res Commun 296:560–567
Preston AM, Gurisik E, Bartley C et al (2009) Reduced endoplasmic reticulum (ER)-to-Golgi protein trafficking contributes to ER stress in lipotoxic mouse beta cells by promoting protein overload. Diabetologia 52:2369–2373. https://doi.org/10.1007/s00125-009-1506-5
Tsvetanova NG (2013) The secretory pathway in control of endoplasmic reticulum homeostasis. Small GTPases 4:28–33. https://doi.org/10.4161/sgtp.22599
van Dis V, Kuijpers M, Haasdijk ED et al (2014) Golgi fragmentation precedes neuromuscular denervation and is associated with endosome abnormalities in SOD1-ALS mouse motor neurons. Acta Neuropathol Commun 2:38. https://doi.org/10.1186/2051-5960-2-38
Fujita Y, Okamoto K (2005) Golgi apparatus of the motor neurons in patients with amyotrophic lateral sclerosis and in mice models of amyotrophic lateral sclerosis. Neuropathology 25:388–394
Gonatas NK, Stieber A, Gonatas JO (2006) Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J Neurol Sci 246:21–30. https://doi.org/10.1016/j.jns.2006.01.019
Ito H, Nakamura M, Komure O et al (2011) Clinicopathologic study on an ALS family with a heterozygous E478G optineurin mutation. Acta Neuropathol 122:223–229. https://doi.org/10.1007/s00401-011-0842-y
Wilson BS, Nuoffer C, Meinkoth JL et al (1994) A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J Cell Biol 125:557–571
Cole NB, Ellenberg J, Song J et al (1998) Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol 140:1–15
Kleijnen MF, Shih AH, Zhou P et al (2000) The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol Cell 6:409–419
Sifers RN, Brashears-Macatee S, Kidd VJ et al (1988) A frameshift mutation results in a truncated alpha 1-antitrypsin that is retained within the rough endoplasmic reticulum. J Biol Chem 263:7330–7335
Grotzke JE, Lu Q, Cresswell P (2013) Deglycosylation-dependent fluorescent proteins provide unique tools for the study of ER-associated degradation. Proc Natl Acad Sci USA 110:3393–3398. https://doi.org/10.1073/pnas.1300328110
Bolte S, Cordelières FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232. https://doi.org/10.1111/j.1365-2818.2006.01706.x
Manders EMM, Verbeek FJ, Aten JA (1993) Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169:375–382. https://doi.org/10.1111/j.1365-2818.1993.tb03313.x
Farg MA, Soo KY, Warraich ST et al (2013) Ataxin-2 interacts with FUS and intermediate-length polyglutamine expansions enhance FUS-related pathology in amyotrophic lateral sclerosis. Hum Mol Genet 22:717–728. https://doi.org/10.1093/hmg/dds479
Muppirala M, Gupta V, Swarup G (2011) Syntaxin 17 cycles between the ER and ERGIC and is required to maintain the architecture of ERGIC and Golgi. Biol Cell 103:333–350. https://doi.org/10.1042/BC20110006
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676
Presley JF, Cole NB, Schroer TA et al (1997) ER-to-Golgi transport visualized in living cells. Nature 389:81–85. https://doi.org/10.1038/38001
Nehls S, Snapp EL, Cole NB et al (2000) Dynamics and retention of misfolded proteins in native ER membranes. Nat Cell Biol 2:288–295. https://doi.org/10.1038/35010558
Nakagomi S, Barsoum MJ, Bossy-Wetzel E et al (2008) A Golgi fragmentation pathway in neurodegeneration. Neurobiol Dis 29:221–231. https://doi.org/10.1016/j.nbd.2007.08.015
Sütterlin C, Hsu P, Mallabiabarrena A, Malhotra V (2002) Fragmentation and dispersal of the pericentriolar Golgi complex is required for entry into mitosis in mammalian cells. Cell 109:359–369
Sugawara T, Nakatsu D, Kii H et al (2012) PKCδ and ε regulate the morphological integrity of the ER—Golgi intermediate compartment (ERGIC) but not the anterograde and retrograde transports via the Golgi apparatus. Biochimica et Biophysica Acta (BBA) Mol Cell Res 1823:861–875. https://doi.org/10.1016/j.bbamcr.2012.01.007
Duellman T, Burnett J, Shin A, Yang J (2015) LMAN1 (ERGIC-53) is a potential carrier protein for matrix metalloproteinase-9 glycoprotein secretion. Biochem Biophys Res Commun 464:685–691. https://doi.org/10.1016/j.bbrc.2015.06.164
Spatuzza C, Renna M, Faraonio R et al (2004) heat shock induces preferential translation of ERGIC-53 and affects its recycling pathway. J Biol Chem 279:42535–42544. https://doi.org/10.1074/jbc.M401860200
Tomás M, Martínez-Alonso E, Ballesta J, Martínez-Menárguez JA (2010) Regulation of ER-Golgi intermediate compartment tubulation and mobility by COPI coats, motor proteins and microtubules. Traffic 11:616–625. https://doi.org/10.1111/j.1600-0854.2010.01047.x
Walker AK, Farg MA, Bye CR et al (2010) Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain 133:105–116. https://doi.org/10.1093/brain/awp267
Oslowski CM, Urano F (2011) Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol 490:71–92. https://doi.org/10.1016/B978-0-12-385114-7.00004-0
Hosokawa N, Tremblay LO, You Z et al (2003) Enhancement of endoplasmic reticulum (ER) degradation of misfolded Null Hong Kong alpha1-antitrypsin by human ER mannosidase I. J Biol Chem 278:26287–26294. https://doi.org/10.1074/jbc.M303395200
Martínez-Menárguez JÁ, Tomás M, Martínez-Martínez N, Martínez-Alonso E (2019) Golgi fragmentation in neurodegenerative diseases: is there a common cause? Cells 8:748
Fan J, Hu Z, Zeng L et al (2008) Golgi apparatus and neurodegenerative diseases. Int J Dev Neurosci 26:523–534
Budnik A, Stephens DJ (2009) ER exit sites—localization and control of COPII vesicle formation. FEBS Lett 583:3796–3803
Yerbury JJ, Ooi L, Dillin A et al (2016) Walking the tightrope: proteostasis and neurodegenerative disease. J Neurochem. https://doi.org/10.1111/jnc.13575
Dinter A, Berger EG (1998) Golgi-disturbing agents. Histochem Cell Biol 109:571–590
Lee TH, Linstedt AD (1999) Osmotically induced cell volume changes alter anterograde and retrograde transport, golgi structure, and COPI dissociation. Mol Biol Cell 10:1445–1462
Hicks SW, Machamer CE (2005) Golgi structure in stress sensing and apoptosis. Biochim Biophys Acta 1744:406–414. https://doi.org/10.1016/j.bbamcr.2005.03.002
Sundaramoorthy V, Sultana JM, Atkin JD (2015) Golgi fragmentation in amyotrophic lateral sclerosis, an overview of possible triggers and consequences. Front Neurosci. https://doi.org/10.3389/fnins.2015.00400
Mourelatos Z, Gonatas NK, Stieber A et al (1996) The Golgi apparatus of spinal cord motor neurons in transgenic mice expressing mutant Cu, Zn superoxide dismutase becomes fragmented in early, preclinical stages of the disease. Proc Natl Acad Sci USA 93:5472–5477
Atkin JD, Farg MA, Soo KY et al (2014) Mutant SOD1 inhibits ER-Golgi transport in amyotrophic lateral sclerosis. J Neurochem 129:190–204. https://doi.org/10.1111/jnc.12493
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13:184–190. https://doi.org/10.1038/ncb0311-184
Hetz C, Thielen P, Matus S et al (2009) XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev 23:2294–2306. https://doi.org/10.1101/gad.1830709
Ito Y, Yamada M, Tanaka H et al (2009) Involvement of CHOP, an ER-stress apoptotic mediator, in both human sporadic ALS and ALS model mice. Neurobiol Dis 36:470–476. https://doi.org/10.1016/j.nbd.2009.08.013
Sasaki S (2010) Endoplasmic reticulum stress in motor neurons of the spinal cord in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 69:346–355. https://doi.org/10.1097/NEN.0b013e3181d44992
Matus S, Lopez E, Valenzuela V et al (2013) Functional contribution of the transcription factor ATF4 to the pathogenesis of amyotrophic lateral sclerosis. PLoS One 8:e66672. https://doi.org/10.1371/journal.pone.0066672
Kiskinis E, Sandoe J, Williams LA et al (2014) Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell 14:781–795. https://doi.org/10.1016/j.stem.2014.03.004
Appenzeller-Herzog C, Hauri H-P (2006) The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci 119:2173–2183. https://doi.org/10.1242/jcs.03019
Fu Y-L, Zhang B, Mu T-W (2019) LMAN1 (ERGIC-53) promotes trafficking of neuroreceptors. Biochem Biophys Res Commun 511:356–362. https://doi.org/10.1016/j.bbrc.2019.02.053
Fu Y-L, Wang Y-J, Mu T-W (2016) Proteostasis maintenance of Cys-loop receptors. Adv Protein Chem Struct Biol 103:1–23. https://doi.org/10.1016/bs.apcsb.2015.11.002
Kaplan A, Spiller KJ, Towne C et al (2014) Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron 81:333–348. https://doi.org/10.1016/j.neuron.2013.12.009
Spiller KJ, Khan T, Dominique MA et al (2019) Reduction of matrix metalloproteinase 9 (MMP-9) protects motor neurons from TDP-43-triggered death in rNLS8 mice. Neurobiol Dis 124:133–140. https://doi.org/10.1016/j.nbd.2018.11.013
Huang B, Wu Q, Zhou H et al (2016) Increased Ubqln2 expression causes neuron death in transgenic rats. J Neurochem 139:285–293. https://doi.org/10.1111/jnc.13748
Suzuki H, Lee K, Matsuoka M (2011) TDP-43-induced death is associated with altered regulation of BIM and Bcl-xL and attenuated by caspase-mediated TDP-43 cleavage. J Biol Chem 286:13171–13183. https://doi.org/10.1074/jbc.M110.197483
Jaarsma D, Haasdijk ED, Grashorn JA et al (2000) Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis 7:623–643. https://doi.org/10.1006/nbdi.2000.0299
Prudencio M, Durazo A, Whitelegge JP, Borchelt DR (2010) An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1. Hum Mol Genet 19:4774–4789. https://doi.org/10.1093/hmg/ddq408
Cannon AD, Zhang Y, Knight J et al (2010) Inducible expression of wild-type TDP-43 is toxic to mouse hippocampal and cortical neurons. Alzheimer’s Dement 6:S225–S226. https://doi.org/10.1016/j.jalz.2010.05.729
Xu Y-F, Gendron TF, Zhang Y-J et al (2010) Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci 30:10851–10859. https://doi.org/10.1523/JNEUROSCI.1630-10.2010
Xia Y, Yan LH, Huang B et al (2014) Pathogenic mutation of UBQLN2 impairs its interaction with UBXD8 and disrupts endoplasmic reticulum-associated protein degradation. J Neurochem 129:99–106. https://doi.org/10.1111/jnc.12606
Kim T-Y, Kim E, Yoon SK, Yoon J-B (2008) Herp enhances ER-associated protein degradation by recruiting ubiquilins. Biochem Biophys Res Commun 369:741–746. https://doi.org/10.1016/j.bbrc.2008.02.086
Schulze A, Standera S, Buerger E et al (2005) The ubiquitin-domain protein HERP forms a complex with components of the endoplasmic reticulum associated degradation pathway. J Mol Biol 354:1021–1027. https://doi.org/10.1016/j.jmb.2005.10.020
Lim PJ, Danner R, Liang J et al (2009) Ubiquilin and p97/VCP bind erasin, forming a complex involved in ERAD. J Cell Biol 187:201–217. https://doi.org/10.1083/jcb.200903024
Nagai A, Kadowaki H, Maruyama T et al (2009) USP14 inhibits ER-associated degradation via interaction with IRE1alpha. Biochem Biophys Res Commun 379:995–1000. https://doi.org/10.1016/j.bbrc.2008.12.182
Chang L, Monteiro MJ (2015) Defective proteasome delivery of polyubiquitinated proteins by ubiquilin-2 proteins containing ALS mutations. PLoS One 10:e0130162. https://doi.org/10.1371/journal.pone.0130162
Nishitoh H, Kadowaki H, Nagai A et al (2008) ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev 22:1451–1464. https://doi.org/10.1101/gad.1640108
Travers KJ, Patil CK, Wodicka L et al (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101:249–258
Author information
Authors and Affiliations
Contributions
All authors contributed to this article. JDA conceived and directed the project. JDA, MH, AMGR, K-YS, and VS designed the experiments. MH, AMGR designed and prepared the UBQLN2 constructs. HS helped to design the UBQLN2 constructs. MH performed and analysed experiments in Neuro2A cells (ER–Golgi transport, Golgi and ERGIC fragmentation, ER stress). AMGR performed and analysed the ERAD assay. MV, BH and SP performed and analysed experiments in human primary neurons. NG and SY performed and analysed experiments in human sections. MH, AMGR and JDA wrote, edited and revised the manuscript. All authors discussed results and commented on the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Halloran, M., Ragagnin, A.M.G., Vidal, M. et al. Amyotrophic lateral sclerosis-linked UBQLN2 mutants inhibit endoplasmic reticulum to Golgi transport, leading to Golgi fragmentation and ER stress. Cell. Mol. Life Sci. 77, 3859–3873 (2020). https://doi.org/10.1007/s00018-019-03394-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03394-w