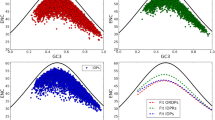
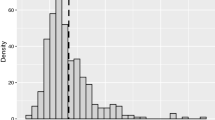
Abstract
Protein-coding nucleic acids exhibit composition and codon biases between sequences coding for intrinsically disordered regions (IDRs) and those coding for structured regions. IDRs are regions of proteins that are folding self-insufficient and which function without the prerequisite of folded structure. Several authors have investigated composition bias or codon selection in regions encoding for IDRs, primarily in Eukaryota, and concluded that elevated GC content is the result of the biased amino acid composition of IDRs. We substantively extend previous work by examining GC content in regions encoding IDRs, from 44 species in Eukaryota, Archaea, and Bacteria, spanning a wide range of GC content. We confirm that regions coding for IDRs show a significantly elevated GC content, even across all domains of life. Although this is largely attributable to the amino acid composition bias of IDRs, we show that this bias is independent of the overall GC content and, most importantly, we are the first to observe that GC content bias in IDRs is significantly different than expected from IDR amino acid composition alone. We empirically find compensatory codon selection that reduces the observed GC content bias in IDRs. This selection is dependent on the overall GC content of the organism. The codon selection bias manifests as use of infrequent, AT-rich codons in encoding IDRs. Further, we find these relationships to be independent of the intrinsic disorder prediction method used, and independent of estimated translation efficiency. These observations are consistent with the previous work, and we speculate on whether the observed biases are causal or symptomatic of other driving forces.






Similar content being viewed by others
References
Dunker AK, Obradovic Z (2001) The protein trinity-linking function and disorder. Nat Biotechnol 19:805–806
Wright PE, Dyson HJ (1999) Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol 293:321–331
Uversky VN, Gillespie JR, Fink AL (2000) Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins 41:415–427
Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6:197–208
Dunker AK, Brown CJ, Lawson JD, Iakoucheva LM, Obradovic Z (2002) Intrinsic disorder and protein function. Biochemistry 41:6573–6582
Xie H, Vucetic S, Iakoucheva LM, Oldfield CJ, Dunker AK, Uversky VN, Obradovic Z (2007) Functional anthology of intrinsic disorder. 1. Biological processes and functions of proteins with long disordered regions. J Proteome Res 6:1882–1898
Peng Z et al (2015) Exceptionally abundant exceptions: comprehensive characterization of intrinsic disorder in all domains of life. Cell Mol Life Sci 72:137–151
Peng Z, Mizianty MJ, Kurgan L (2014) Genome-scale prediction of proteins with long intrinsically disordered regions. Proteins 82:145–158
Xue B, Dunker AK, Uversky VN (2012) Orderly order in protein intrinsic disorder distribution: disorder in 3500 proteomes from viruses and the three domains of life. J Biomol Struct Dyn 30:137–149
Pancsa R, Tompa P (2012) Structural disorder in eukaryotes. PLoS One 7:e34687
Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT (2004) Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 337:635–645
Tompa P (2012) Intrinsically disordered proteins: a 10-year recap. Trends Biochem Sci 37:509–516
Dosztanyi Z, Csizmok V, Tompa P, Simon I (2005) The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J Mol Biol 347:827–839
Walsh I, Martin AJ, Di Domenico T, Tosatto SC (2012) ESpritz: accurate and fast prediction of protein disorder. Bioinformatics 28:503–509
Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK (2001) Sequence complexity of disordered protein. Proteins 42:38–48
Peng K, Radivojac P, Vucetic S, Dunker AK, Obradovic Z (2006) Length-dependent prediction of protein intrinsic disorder. BMC Bioinform 7:208
Meng F, Uversky VN, Kurgan L (2017) Comprehensive review of methods for prediction of intrinsic disorder and its molecular functions. Cell Mol Life Sci 74:3069–3090
Lieutaud P, Ferron F, Uversky AV, Kurgan L, Uversky VN, Longhi S (2016) How disordered is my protein and what is its disorder for? A guide through the “dark side” of the protein universe. Intrinsically Disord Proteins 4:e1259708
Romero PR et al (2006) Alternative splicing in concert with protein intrinsic disorder enables increased functional diversity in multicellular organisms. Proc Natl Acad Sci USA 103:8390–8395
Homma K, Noguchi T, Fukuchi S (2016) Codon usage is less optimized in eukaryotic gene segments encoding intrinsically disordered regions than in those encoding structural domains. Nucleic Acids Res 44:10051–10061
Zhou M, Wang T, Fu J, Xiao G, Liu Y (2015) Nonoptimal codon usage influences protein structure in intrinsically disordered regions. Mol Microbiol 97:974–987
Peng Z, Uversky VN, Kurgan L (2016) Genes encoding intrinsic disorder in Eukaryota have high GC content. Intrinsically Disord Proteins 4:e1262225
Basile W, Sachenkova O, Light S, Elofsson A (2017) High GC content causes orphan proteins to be intrinsically disordered. PLoS Comput Biol 13:e1005375
Yruela I, Contreras-Moreira B (2013) Genetic recombination is associated with intrinsic disorder in plant proteomes. BMC Genom 14:772
Pavlovic-Lazetic GM, Mitic NS, Kovacevic JJ, Obradovic Z, Malkov SN, Beljanski MV (2011) Bioinformatics analysis of disordered proteins in prokaryotes. BMC Bioinform 12:66
Bernardi G (1993) The vertebrate genome: isochores and evolution. Mol Biol Evol 10:186–204
Yin H, Wang G, Ma L, Yi SV, Zhang Z (2016) What signatures dominantly associate with gene age? Genome Biol Evol 8:3083–3089
Amit M et al (2012) Differential GC content between exons and introns establishes distinct strategies of splice-site recognition. Cell Rep 1:543–556
Romero P, Obradovic Z, Li X, Garner EC, Brown CJ, Dunker AK (2001) Sequence complexity of disordered protein. Proteins Struct Funct Bioinform 42:38–48
Cannarozzi G et al (2010) A role for codon order in translation dynamics. Cell 141:355–367
Pruitt KD et al (2009) The consensus coding sequence (CCDS) project: identifying a common protein-coding gene set for the human and mouse genomes. Genome Res 19:1316–1323
UniProt Consortium (2012) Reorganizing the protein space at the Universal Protein Resource (UniProt). Nucleic Acids Res 40:D71–D75
Kanz C et al (2005) The EMBL nucleotide sequence database. Nucleic Acids Res 33:D29–D33
Peng ZL, Kurgan L (2012) Comprehensive comparative assessment of in silico predictors of disordered regions. Curr Protein Pept Sci 13:6–18
Walsh I, Giollo M, Di Domenico T, Ferrari C, Zimmermann O, Tosatto SC (2015) Comprehensive large-scale assessment of intrinsic protein disorder. Bioinformatics 31:201–208
Piovesan D et al (2016) DisProt 7.0: a major update of the database of disordered proteins. Nucleic Acids Res D1:D219–D227
Peng, Z. and Kurgan, L. (2012). On the complementarity of the consensus-based disorder prediction. In: Pacific symposium on biocomputing, pp 176–187
Fan X, Kurgan L (2014) Accurate prediction of disorder in protein chains with a comprehensive and empirically designed consensus. J Biomol Struct Dyn 32:448–464
Na I, Meng F, Kurgan L, Uversky VN (2016) Autophagy-related intrinsically disordered proteins in intra-nuclear compartments. Mol BioSyst 12:2798–2817
Meng F, Na I, Kurgan L, Uversky VN (2016) Compartmentalization and functionality of nuclear disorder: intrinsic disorder and protein–protein interactions in intra-nuclear compartments. Int J Mol Sci 17:24
Peng Z, Oldfield CJ, Xue B, Mizianty MJ, Dunker AK, Kurgan L, Uversky VN (2014) A creature with a hundred waggly tails: intrinsically disordered proteins in the ribosome. Cell Mol Life Sci 71:1477–1504
Hu G, Wu Z, Wang K, Uversky VN, Kurgan L (2016) Untapped potential of disordered proteins in current druggable human proteome. Curr Drug Targets 17:1198–1205
Wang C, Uversky VN, Kurgan L (2016) Disordered nucleiome: abundance of intrinsic disorder in the DNA- and RNA-binding proteins in 1121 species from Eukaryota, Bacteria and Archaea. Proteomics 16:1486–1498
Di Domenico T, Walsh I, Martin AJM, Tosatto SCE (2012) MobiDB: a comprehensive database of intrinsic protein disorder annotations. Bioinformatics 28:2080–2081
Potenza E, Di Domenico T, Walsh I, Tosatto SC (2015) MobiDB 2.0: an improved database of intrinsically disordered and mobile proteins. Nucleic Acids Res 43:D315–D320
Oates ME et al (2013) D(2)P(2): database of disordered protein predictions. Nucleic Acids Res 41:D508–D516
Li X, Romero P, Rani M, Dunker AK, Obradovic Z (1999) Predicting protein disorder for N-, C-, and internal regions. Genome Inform Ser Workshop Genome Inform 10:30–40
Bennetzen JL, Hall BD (1982) Codon selection in yeast. J Biol Chem 257:3026–3031
Friberg MT, Gonnet P, Barral Y, Schraudolph NN, Gonnet GH (2006) Measures of codon bias in yeast, the tRNA pairing index and possible DNA repair mechanisms. In: Bucher P, Moret B (eds) Algorithms in bioinformatics. WABI 2006. Lecture Notes in Computer Science, vol 4175. Springer, Berlin, Heidelberg
Guo F-B, Ye Y-N, Zhao H-L, Lin D, Wei W (2012) Universal pattern and diverse strengths of successive synonymous codon bias in three domains of life, particularly among prokaryotic genomes. DNA Res Int J Rapid Publ Rep Genes Genomes 19:477–485
Reis MD, Savva R, Wernisch L (2004) Solving the riddle of codon usage preferences: a test for translational selection. Nucleic Acids Res 32:5036–5044
Novoa EM, Ribas de Pouplana L (2012) Speeding with control: codon usage, tRNAs, and ribosomes. Trends Genet 28:574–581
Petersen J, Eriksson SK, Harryson P, Pierog S, Colby T, Bartels D, Rohrig H (2012) The lysine-rich motif of intrinsically disordered stress protein CDeT11-24 from Craterostigma plantagineum is responsible for phosphatidic acid binding and protection of enzymes from damaging effects caused by desiccation. J Exp Bot 63:4919–4929
Botting CH, Talbot P, Paytubi S, White MF (2010) Extensive lysine methylation in hyperthermophilic crenarchaea: potential implications for protein stability and recombinant enzymes. Archaea 2010:106341
Varadi M, Zsolyomi F, Guharoy M, Tompa P (2015) Functional advantages of conserved intrinsic disorder in RNA-binding proteins. PLoS One 10:e0139731
Uversky VN (2017) Protein intrinsic disorder-based liquid–liquid phase transitions in biological systems: complex coacervates and membrane-less organelles. Adv Colloid Interface Sci 239:97–114
Siddiqui KS, Cavicchioli R (2006) Cold-adapted enzymes. Annu Rev Biochem 75:403–433
Acknowledgements
This research was supported in part by the National Science Foundation Grant 1617369 and the Robert J. Mattauch Endowment from Virginia Commonwealth University to L.K.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oldfield, C.J., Peng, Z., Uversky, V.N. et al. Codon selection reduces GC content bias in nucleic acids encoding for intrinsically disordered proteins. Cell. Mol. Life Sci. 77, 149–160 (2020). https://doi.org/10.1007/s00018-019-03166-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03166-6