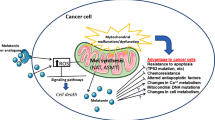
Abstract
The long-recognized fact that oxidative stress within mitochondria is a hallmark of mitochondrial dysfunction has stimulated the development of mitochondria-targeted antioxidant therapies. Melatonin should be included among the pharmacological agents able to modulate mitochondrial functions in cancer, given that a number of relevant melatonin-dependent effects are triggered by targeting mitochondrial functions. Indeed, melatonin may modulate the mitochondrial respiratory chain, thus antagonizing the cancer highly glycolytic bioenergetic pathway of cancer cells. Modulation of the mitochondrial respiratory chain, together with Ca2+ release and mitochondrial apoptotic effectors, may enhance the spontaneous or drug-induced apoptotic processes. Given that melatonin may efficiently counteract the Warburg effect while stimulating mitochondrial differentiation and mitochondrial-based apoptosis, it is argued that the pineal neurohormone could represent a promising new perspective in cancer treatment strategy.

Similar content being viewed by others
References
Pagliarini DJ, Rutter J (2013) Hallmarks of a new era in mitochondrial biochemistry. Genes Dev 27:2615–2627. doi:10.1101/gad.229724.113
Wallace DC (2012) Mitochondria and cancer. Nat Rev Cancer 12:685–698. doi:10.1038/nrc3365
Sullivan LB, Chandel NS (2014) Mitochondrial reactive oxygen species and cancer. Cancer Metab 2:17. doi:10.1186/2049-3002-2-17
Moldoveanu T, Follis AV, Kriwacki RW, Green DR (2014) Many players in BCL-2 family affairs. Trends Biochem Sci 39:101–111. doi:10.1016/j.tibs.2013.12.006
Duchen MR (2000) Mitochondria and calcium: from cell signalling to cell death. J Physiol 15:529
Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES (2008) Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454:766–770. doi:10.1038/nature07107
Hamalainen RH, Manninen T, Koivumaki H, Kislin M, Otonkoski T, Suomalainen A (2013) Tissue- and cell-type-specific manifestations of heteroplasmic mtDNA 3243A>G mutation in human induced pluripotent stem cell-derived disease model. Proc Natl Acad Sci USA 110(38):E3622–E3630. doi:10.1073/pnas.1311660110
Nunnari J, Suomalainen A (2012) Mitochondria: in sickness and in health. Cell 148:1145–1159. doi:10.1016/j.cell.2012.02.035
Tan DX, Manchester LC, Qin L, Reiter RJ (2016) Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci 17(12):E2124. doi:10.3390/ijms17122124
Raza H, John A, Brown EM, Benedict S, Kambal A (2008) Alterations in mitochondrial respiratory functions, redox metabolism and apoptosis by oxidant 4-hydroxynonenal and antioxidants curcumin and melatonin in PC12 cells. Toxicol Appl Pharmacol 226(2):161–168
Acuna-Castroviejo D, Escames G, Venegas C, Diaz-Casado ME, Lima-Cabello E, Lopez LC, Rosales-Corral S, Tan DX, Reiter RJ (2014) Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71:2997–3025. doi:10.1007/s00018-014-1579-2
Coelho LA, Peres R, Amaral FG, Reiter RJ, Cipolla-Neto J (2015) Daily differential expression of melatonin-related genes and clock genes in rat cumulus-oocyte complex: changes after pinealectomy. J Pineal Res 58:490–499. doi:10.1111/jpi.12234
Sakaguchi K, Itoh MT, Takahashi N, Tarumi W, Ishizuka B (2013) The rat oocyte synthesises melatonin. Reprod Fertil Dev 25:674–682. doi:10.1071/RD12091
Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J (2006) Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology 228:333–343. doi:10.1016/j.tox.2006.09.018
Horbay R, Bilyy R (2016) Mitochondrial dynamics during cell cycling. Apoptosis 21:1327–1335. doi:10.1007/s10495-016-1295-5
Neufeld-Cohen A, Robles MS, Aviram R, Manella G, Adamovich Y, Ladeuix B, Nir D, Rousso-Noori L, Kuperman Y, Golik M, Mann M, Asher G (2016) Circadian control of oscillations in mitochondrial rate-limiting enzymes and nutrient utilization by PERIOD proteins. Proc Natl Acad Sci USA 113:E1673–E1682. doi:10.1073/pnas.1519650113
Hardeland R, Madrid JA, Tan DX, Reiter RJ (2012) Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res 52:139–166. doi:10.1111/j.1600-079X.2011.00934.x
Kandalepas PC, Mitchell JW, Gillette MU (2016) Melatonin signal transduction pathways require E-box-mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLoS One 11:e015. doi:10.1371/journal.pone.0157824
Hsiao CW, Peng TI, Peng AC, Reiter RJ, Tanaka M, Lai YK, Jou MJ (2013) Long-term Aβ exposure augments mCa2+-independent mROS-mediated depletion of cardiolipin for the shift of a lethal transient mitochondrial permeability transition to its permanent mode in NARP cybrids: a protective targeting of melatonin. J Pineal Res 54:107–125. doi:10.1111/jpi.12004
Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12:2245–2256. doi:10.1091/mbc.12.8.2245
Suwanjang W, Abramov AY, Charngkaew K, Govitrapong P, Chetsawang B (2016) Melatonin prevents cytosolic calcium overload, mitochondrial damage and cell death due to toxically high doses of dexamethasone-induced oxidative stress in human neuroblastoma SH-SY5Y cells. Neurochem Int 97:34–41. doi:10.1016/j.neuint.2016.05.003
Pei H, Du J, Song X, He L, Zhang Y, Li X, Qiu C, Zhang Y, Hou J, Feng J, Gao E, Li D, Yang Y (2016) Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic Biol Med 97:408–417. doi:10.1016/j.freeradbiomed.2016.06.015
Coto-Montes A, Boga JA, Rosales-Corral S, Fuentes-Broto L, Tan DX, Reiter RJ (2012) Role of melatonin in the regulation of autophagy and mitophagy: a review. Mol Cell Endocrinol 361:12–23. doi:10.1016/j.mce.2012.04.009
Kang JW, Hong JM, Lee SM (2016) Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J Pineal Res 60:383–393. doi:10.1111/jpi.12319
Reiter RJ, Tan DX, Qi W, Manchester LC, Karbownik M, Calvo JR (2000) Pharmacology and physiology of melatonin in the reduction of oxidative stress in vivo. Biol Signals Recept 9:160–171
Kotler M, Rodriguez C, Sainz RM, Antolin I, Menendez-Pelaez A (1998) Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res 24(2):83–89
Urata Y, Honma S, Goto S, Todoroki S, Iida T, Cho S, Honma K, Kondo T (1999) Melatonin induces gamma-glutamylcysteine synthetase mediated by activator protein-1 in human vascular endothelial cells. Free Radic Biol Med 27:838–847
Okatani Y, Wakatsuki A, Kaneda C (2000) Melatonin increases activities of glutathione peroxidase and superoxide dismutase in fetal rat brain. J Pineal Res 28(2):89–96. doi:10.1034/j.1600-079X.2001.280204.x
Cuzzocrea S, Costantino G, Caputi AP (1998) Protective effect of melatonin on cellular energy depletion mediated by peroxynitrite and poly (ADP-ribose) synthetase activation in a non-septic shock model induced by zymosan in the rat. J Pineal Res 25(2):78–85
Martín M, Macías M, Escames G, Reiter RJ, Agapito MT, Ortiz GG, Acuña-Castroviejo D (2000) Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res 28:242–248. doi:10.1034/j.1600-079X.2000.280407.x
Liu H, Kehrer JP (1996) The reduction of glutathione disulfide produced by t-butyl hydroperoxide in respiring mitochondria. Free Radic Biol Med 20(3):433–442
Martin M, Macias M, Escames G, Leon J, Acuna-Castroviejo D (2000) Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 14:1677–1679. doi:10.1096/fj.99-0865fje
López A, García JA, Escames G, Venegas C, Ortiz F, López LC, Acuña-Castroviejo D (2009) Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res 46:188–198. doi:10.1111/j.1600-079X.2008.00647.x
Martin M, Macias M, Leon J, Escames G, Khaldy H, Acuna-Castroviejo D (2002) Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol 34(4):348–357. doi:10.1016/S1357-2725(01)00138-8
Leon J, Acuña-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ (2004) Melatonin and mitochondrial function. Life Sci 75(7):765–790. doi:10.1016/j.lfs.2004.03.003
Acuña-Castroviejo D, Escames G, López LC, Hitos AB, León J (2005) Melatonin and nitric oxide: two required antagonists for mitochondrial homeostasis. Endocrine 27:159–168. doi:10.1385/ENDO:27:2:159
Prunet-Marcassus BL, Ambid N, Viguerie-Bascands L, Pénicaud L, Casteilla L (2001) Evidence for a direct effect of melatonin on mitochondrial genome expression of Siberian hamster brown adipocytes. J Pineal Res 30:108–115. doi:10.1034/j.1600-079X.2001.300206.x
Acuña-Castroviejo D, Escames G, López León J, Carazo A, Khaldy H (2003) Mitochondrial regulation by melatonin and its metabolites. Adv Exp Med Biol 527:549–557
Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N (2005) A mutation in the SDHC gene of complex II increases oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Res 65:203–209
Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K (1998) A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature 394:694–697. doi:10.1038/29331
Warburg O (1926) Ǘber den Stoffwechsel der Tumoren. Springer, Berlin
Garber K (2004) Energy boost: the Warburg effect returns in a new theory of cancer. J Natl Cancer Inst 96:1805–1806. doi:10.1093/jnci/96.24.1805
Hsu PP, Sabatini DM (2008) Cancer cell metabolism: Warburg and beyond. Cell 134:703–707. doi:10.1016/j.cell.2008.08.021
Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL (2003) Overexpression of Glut-1 and increased metabolism in tumours are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 97:1015–1024. doi:10.1002/cncr.11159
Kroemer G, Pouyssegur J (2008) Tumour cell metabolism: cancer’s Achilles’ heel. Cancer Cell 13:472–482. doi:10.1016/j.ccr.2008.05.005
Crabtree HG (1929) Observations on the carbohydrate metabolism of tumours. Biochem J 23:536–545
Pfeiffer T, Schuster S, Bonhoeffer S (2001) Cooperation and competition in the evolution of ATP-producing pathways. Science 292:504–507. doi:10.1126/science.1058079
Postovit LM, Adams MA, Lash GE et al (2002) Oxygen-mediated regulation of tumor cell invasiveness. Involvement of a nitric oxide signaling pathway. J Biol Chem 277:35730–35737. doi:10.1074/jbc.M204529200
Lobo C, Ruiz-Bellido MA, Aledo JC, Márquez J, Núñez De Castro I, Alonso FJ (2000) Inhibition of glutaminase expression by antisense mRNA decreases growth and tumorigenicity of tumour cells. Biochem J 348:257–261. doi:10.1042/bj3480257
Fantin VR, St-Pierre J, Leder P (2006) Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9:425–434. doi:10.1016/j.ccr.2006.04.023
Krieg RC, Knuechel R, Schiffmann E, Liotta LA, Petricoin EF III, Herrmann PC (2004) Mitochondrial proteome: cancer-altered metabolism associated with cytochrome c oxidase subunit level variation. Proteomics 4:2789–2795. doi:10.1002/pmic.200300796
Schomack PA, Gilles RJ (2003) Contributions of cell metabolism and H+ diffusion to the acidic pH of tumours. Neoplasia 5:135–145
Smith TA (2001) The rate-limiting step for tumor [18F]fluoro-2-deoxy-d-glucose (FDG) incorporation. Nucl Med Biol 28:1–4. doi:10.1016/S0969-8051(00)00177-3
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60:916–921
Soderberg K, Nissinen E, Bakay B (1980) The energy charge in wild-type and respiration deficient Chinese hamster cell mutants. J Cell Physiol 103:169–172. doi:10.1002/jcp.1041030121
Gilbert DL, Colton CA (1999) An overview of reactive oxygen species. In: Gilbert DL, Colton CA (eds) Reactive oxygen species in biological systems. Kluwer Academic-Plenum Publishers, New York
Wenger RH (2000) Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol 203:1253–1263
Burdon RH (1996) Control of cell proliferation by reactive oxygen species. Biochem Soc Trans 24:1028–1032
Nose K (2000) Role of reactive oxygen species in the regulation of physiological functions. Biol Pharm Bull 23:897–903
Enns GM (2003) The contribution of mitochondria to common disorders. Mol Genet Metab 80:11–26. doi:10.1016/j.ymgme.2003.08.009
Gatenby RA, Gawlinski ET (1996) A reaction-diffusion model of cancer invasion. Cancer Res 56:5745–5753
Mazurek S, Grimm H, Boschek CB, Vaupel P, Eigenbrodt E (2002) Pyruvate kinase type M2: a crossroad in the tumor metabolome. Br J Nutr 87(Suppl 1):S23–S29
Richardson AD, Yang C, Osterman A, Smith JW (2008) Central carbon metabolism in the progression of mammary carcinoma. Breast Cancer Res Treat 110:297–307. doi:10.1007/s10549-007-9732-3
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899. doi:10.1038/nrc1478
Menendez JA, Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7:763–777. doi:10.1038/nrc2222
Parlo RA, Coleman PS (1984) Enhanced rate of citrate export from cholesterol-rich hepatoma mitochondria. The truncated Krebs cycle and other metabolic ramifications of mitochondrial membrane cholesterol. J Biol Chem 259:9997–10003
Menendez JA, Colomer R, Lupu R (2005) Why does tumour-associated fatty acid synthase (oncogenic antigen 519) ignore dietary fatty acids? Med Hypotheses 64:342–349. doi:10.1016/j.mehy.2004.07.022
Lupu R, Menendez JA (2006) Pharmacological inhibitors of fatty acid synthase (FASN)-catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? Curr Pharm Biotechnol 7:483–493. doi:10.2174/138920106779116928
Pollak M (2013) Potential applications for biguanides in oncology. J Clin Invest 123:3693–3700. doi:10.1172/JCI67232
D’Anselmi F, Valerio M, Cucina A, Galli L, Proietti S, Dinicola S, Pasqualato A, Manetti C, Ricci G, Giuliani A, Bizzarri M (2011) Metabolism and cell shape in cancer: a fractal analysis. Int J Biochem Cell Biol 3:1052–1058. doi:10.1016/j.biocel.2010.05.002
Alirol E, Martinou JC (2006) Mitochondria and cancer: is there a morphological connection? Oncogene 25:4706–4716. doi:10.1038/sj.onc.1209600
Vega-Naredo I, Loureiro R, Mesquita KA, Barbosa IA, Tavares LC, Branco AF, Erickson JR, Holy J, Perkins EL, Carvalho RA, Oliveira PJ (2014) Mitochondrial metabolism directs stemness and differentiation in P19 embryonal carcinoma stem cells. Cell Death Differ 21:1560–1574. doi:10.1038/cdd.2014.66
Loureiro R, Magalhães-Novais S, Mesquita KA, Baldeiras I, Sousa IS, Tavares LC, Barbosa IA, Oliveira PJ, Vega-Naredo I (2015) Melatonin antiproliferative effects require active mitochondrial function in embryonal carcinoma cells. Oncotarget 6:17081–17096. doi:10.18632/oncotarget.4012
Scott AE, Cosma GN, Frank AA, Wells RL, Gardner HS Jr (2001) Disruption of mitochondrial respiration by melatonin in MCF-7 cells. Toxicol Appl Pharmacol 171:149–156. doi:10.1006/taap.2000.9115
Wang BQ, Yang QH, Xu RK, Xu JN (2013) Elevated levels of mitochondrial respiratory complexes activities and ATP production in 17-β-estradiol-induced prolactin-secretory tumor cells in male rats are inhibited by melatonin in vivo and in vitro. Chin Med J (Engl) 126:4724–4730
Tutton PJ, Barkla DH (1977) Cytotoxicity of 5,6-dihydroxytryptamine in dimethylhydrazine-induced carcinomas of rat colon. Cancer Res 37:1241–1244
Klemm HP, Baumgarten HG, Schlossberger HG (1980) Polarographic measurements of spontaneous and mitochondria-promoted oxidation of 5,6- and 5,7-dihydroxytryptamine. J Neurochem 35:1400–1408
Perdomo J, Cabrera J, Estévez F, Loro J, Reiter RJ, Quintana J (2013) Melatonin induces apoptosis through a caspase-dependent but reactive oxygen species-independent mechanism in human leukemia Molt-3 cells. J Pineal Res 55:195–206. doi:10.1111/jpi.12062
Zhang HM, Zhang Y, Zhang BX (2011) The role of mitochondrial complex III in melatonin-induced ROS production in cultured mesangial cells. J Pineal Res 50:78–82. doi:10.1111/j.1600-079X.2010.00815.x
Shellard SA, Whelan RD, Hill BT (1989) Growth inhibitory and cytotoxic effects of melatonin and its metabolites on human tumour cell lines in vitro. Br J Cancer 60:288–290
Sakano K, Oikawa S, Hiraku Y, Kawanishi S (2004) Oxidative DNA damage induced by a melatonin metabolite, 6-hydroxymelatonin, via a unique non-O-quinone type of redox cycle. Biochem Pharmacol 68:1869–1878. doi:10.1016/j.bcp.2004.06.016
Liu L, Xu Y, Reiter RJ (2013) Melatonin inhibits the proliferation of human osteosarcoma cell line MG-63. Bone 55:432–438. doi:10.1016/j.bone.2013.02.021
Hong Y, Won J, Lee Y, Lee S, Park K, Chang KT (2014) Melatonin treatment induces interplay of apoptosis, autophagy, and senescence in human colorectal cancer cells. J Pineal Res 56:264–274. doi:10.1111/jpi.12119
Zhang H, Zhang HM, Wu LP, Tan DX, Kamat A, Li YQ, Katz MS, Abboud HE, Reiter RJ, Zhang BX (2011) Impaired mitochondrial complex III and melatonin responsive reactive oxygen species generation in kidney mitochondria of db/db mice. J Pineal Res 51:338–344. doi:10.1111/j.1600-079X.2011.00894.x
Sousa SC, Castilho RF (2005) Protective effect of melatonin on rotenone plus Ca2+-induced mitochondrial oxidative stress and PC12 cell death. Antioxid Redox Signal 7:1110–1116. doi:10.1089/ars.2005.7.1110
Reyes-Toso CF, Rebagliati IR, Ricci CR, Linares LM, Albornoz LE, Cardinali DP, Zaninovich A (2006) Effect of melatonin treatment on oxygen consumption by rat liver mitochondria. Amino Acids 31:299–302. doi:10.1007/s00726-005-0280-z
Zavodnik IB, Lapshina EA, Cheshchevik VT, Dremza IK, Kujawa J, Zabrodskaya SV, Reiter RJ (2011) Melatonin and succinate reduce rat liver mitochondrial dysfunction in diabetes. J Physiol Pharmacol 62:421–427
Poeggeler B, Sambamurti K, Siedlak SL, Perry G, Smith MA, Pappolla MA (2010) A novel endogenous indole protects rodent mitochondria and extends rotifer lifespan. PLoS One 5:e10206. doi:10.1371/journal.pone.0010206
Okatani Y, Wakatsuki A, Reiter RJ, Miyahara Y (2002) Hepatic mitochondrial dysfunction in senescence-accelerated mice: correction by long-term, orally administered physiological levels of melatonin. J Pineal Res 33:127–133. doi:10.1034/j.1600-079X.2002.02109.x
Proietti S, Cucina A, Reiter RJ, Bizzarri M (2013) Molecular mechanisms of melatonin’s inhibitory actions on breast cancers. Cell Mol Life Sci 70:2139–2157. doi:10.1007/s00018-012-1161-8
Mao L, Dauchy RT, Blask DE, Dauchy EM, Slakey LM, Brimer S, Yuan L, Xiang S, Hauch A, Smith K, Frasch T, Belancio VP, Wren MA, Hill SM (2016) Melatonin suppression of aerobic glycolysis (Warburg effect), survival signalling and metastasis in human leiomyosarcoma. J Pineal Res 60:167–177. doi:10.1111/jpi.12298
Sanchez-Sanchez AM, Antolin I, Puente-Moncada N, Suarez S, Gomez-Lobo M, Rodriguez C, Martin V (2015) Melatonin cytotoxicity is associated to warburg effect inhibition in ewing sarcoma cells. PLoS One 10:e0135420. doi:10.1371/journal.pone.0135420
Stubbs M, Griffiths JR (2010) The altered metabolism of tumors: HIF-1 and its role in the Warburg effect. Adv Enzym Regul 50:44–55. doi:10.1016/j.advenzreg.2009.10.027
Resende RR, Adhikari A, da Costa JL, Lorencon E, Ladeira MS, Guatimosim S, Kihara AH, Ladeira LO (2010) Influence of spontaneous calcium events on cell-cycle progression in embryonal carcinoma and adult stem cells. Biochim Biophys Acta 1803:246–260. doi:10.1016/j.bbamcr.2009.11.008
Dai J, Inscho EW, Yuan L, Hill SM (2002) Modulation of intracellular calcium and calmodulin by melatonin in MCF-7 human breast cancer cells. J Pineal Res 32:112–119. doi:10.1034/j.1600-079x.2002.1844.x
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Dejean LM, Martinez-Caballero S, Manon S, Kinnally KW (2006) Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim Biophys Acta 1762:191–201. doi:10.1016/j.bbadis.2005.07.002
Fesik SW, Shi Y (2001) Controlling the caspases”. Science 294:1477–1478. doi:10.1126/science.1062236
Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ (2003) Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci 60:1407–1426. doi:10.1007/s00018-003-2319-1
Proietti S, Cucina A, Dobrowolny G, D’Anselmi F, Dinicola S, Masiello MG, Pasqualato A, Palombo A, Morini V, Reiter RJ, Bizzarri M (2014) Melatonin down-regulates MDM2 gene expression and enhances p53 acetylation in MCF-7 cells. J Pineal Res 57:120–129. doi:10.1111/jpi.12150
Bizzarri M, Proietti S, Cucina A, Reiter RJ (2013) Molecular mechanisms of the pro-apoptotic actions of melatonin in cancer: a review. Expert Opin Ther Targets 17:1483–1496. doi:10.1517/14728222.2013.834890
Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K (2005) Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J 24:1375–1386. doi:10.1038/sj.emboj.7600614
Cucina A, Proietti S, D’Anselmi F, Coluccia P, Dinicola S, Frati L, Bizzarri M (2009) Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J Pineal Res 46:172–180. doi:10.1111/j.1600-079X.2008.00645.x
Galano A, Tan DX, Reiter RJ (2011) Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res 51:1–16. doi:10.1111/j.1600-079X.2011.00916.x
Büyükavci M, Ozdemir O, Buck S, Stout M, Ravindranath Y, Savaşan S (2006) Melatonin cytotoxicity in human leukemia cells: relation with its pro-oxidant effect. Fundam Clin Pharmacol 20:73–79. doi:10.1111/j.1472-8206.2005.00389.x
Osseni RA, Rat P, Bogdan A, Warnet JM, Touitou Y (2000) Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci 68:387–399. doi:10.1016/S0024-3205(00)00955-3
Matés JM, Segura JA, Alonso FJ, Márquez J (2008) Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol 82:273–299. doi:10.1007/s00204-008-0304-z
Sánchez-Sánchez AM, Martín V, García-Santos G, Rodríguez-Blanco J, Casado-Zapico S, Suarez-Garnacho S, Antolín I, Rodriguez C (2011) Intracellular redox state as determinant for melatonin antiproliferative vs. cytotoxic effects in cancer cells. Free Radic Res 45:1333–1341. doi:10.3109/10715762.2011.623700
Picard M, Wallace DC, Burelle Y (2016) The rise of mitochondria in medicine. Mitochondrion 30:105–116. doi:10.1016/j.mito.2016.07.003
Wang W, Karamanlidis G, Tian R (2016) Novel targets for mitochondrial medicine. Sci Transl Med 8:326rv3. doi:10.1126/scitranslmed.aac7410
Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646. doi:10.1038/sj.onc.1209597
Dwarakanath BS (2009) Cytotoxicity, radiosensitization, and chemosensitization of tumor cells by 2-deoxy-d-glucose in vitro. J Cancer Res Ther 5(Suppl 1):S27–S31. doi:10.4103/0973-1482.55137
Cardaci S, Desideri E, Ciriolo MR (2012) Targeting aerobic glycolysis: 3-bromopyruvate as a promising anticancer drug. J Bioenerg Biomembr 44:17–29. doi:10.1007/s10863-012-9422-7
Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV (2010) Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA 107:2037–2042. doi:10.1073/pnas.0914433107
Pacini N, Borziani F (2016) Oncostatic-cytoprotective effect of melatonin and other bioactive molecules: a common target in mitochondrial respiration. Int J Mol Sci 17:341. doi:10.3390/ijms17030341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Proietti, S., Cucina, A., Minini, M. et al. Melatonin, mitochondria, and the cancer cell. Cell. Mol. Life Sci. 74, 4015–4025 (2017). https://doi.org/10.1007/s00018-017-2612-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2612-z