Abstract
The inflammasome adapter ASC links activated inflammasome sensors to the effector molecule pro-caspase-1. Recruitment of pro-caspase-1 to ASC promotes the autocatalytic activation of caspase-1, which leads to the release of pro-inflammatory cytokines, such as IL-1β. Upon triggering of inflammasome sensors, ASC assembles into large helical fibrils that interact with each other serving as a supramolecular signaling platform termed the ASC speck. Alternative splicing, post-translational modifications of ASC, as well as interaction with other proteins can perturb ASC function. In several inflammatory diseases, ASC specks can be found in the extracellular space and its presence correlates with poor prognosis. Here, we review the role of ASC in inflammation, and focus on the structural mechanisms that lead to ASC speck formation, the regulation of ASC function during inflammasome assembly, and the importance of ASC specks in disease.




Similar content being viewed by others
Abbreviations
- aa:
-
Amino acid
- AIM2:
-
Absent in melanoma 2
- APAF-1:
-
Apoptotic protease-activating factor 1
- ASC:
-
Apoptosis-associated speck-like protein containing a CARD
- CAPS:
-
Cryopyrin-associated periodic syndrome
- CARD:
-
Caspase-recruitment domain
- COP:
-
CARD-only protein
- DAMP:
-
Damage-associated molecular pattern
- DD:
-
Death domain
- DED:
-
Death effector domain
- E1:
-
Ubiquitin-activating enzyme
- E2:
-
Ubiquitin-conjugating enzyme
- E3:
-
Ubiquitin-ligation enzyme
- EPSP:
-
Electrostatic potential surface patch
- FCAS:
-
Familial cold autoinflammatory syndrome
- FMF:
-
Familial Mediterranean fever
- HIN200:
-
Hematopoietic interferon-inducible nuclear antigens with 200 amino acid repeats
- HOIL-1:
-
Heme-oxidized IRP2 ubiquitin ligase-1
- HOIP:
-
HOIL-1 interacting protein
- IKK:
-
IκB kinase
- IL:
-
Interleukin
- Jnk:
-
c-Jun N-terminal kinase
- LPS:
-
Lipopolysaccharide
- LUBAC:
-
Linear ubiquitin chain assembly complex
- MAVS:
-
Mitochondrial antiviral-signaling
- MEFV:
-
Mediterranean fever
- MV:
-
Myxoma virus
- NAIP:
-
NLR family apoptosis inhibitory protein
- NF-κB:
-
Nuclear factor kappa-light-chain enhancer of activated B-cells
- NLR:
-
Nucleotide-binding oligomerization domain (NOD)-like receptor
- NLRC:
-
NOD-, LRR-, CARD-containing
- NLRP:
-
NOD-, leucine-rich repeat (LRR)-, PYD-containing
- NOD:
-
Nucleotide oligomerization domain
- NOMID:
-
Neonatal-onset multisystem inflammatory disorder
- PAMP:
-
Pathogen-associated molecular pattern
- POP:
-
Pyrin-only protein
- PRR:
-
Pattern-recognition receptor
- PTM:
-
Post-translational modification
- PYCARD:
-
PYD and CARD domain containing
- PYD:
-
Pyrin domain
- Pydc:
-
Pyrin domain containing
- RIPK2:
-
Receptor-interacting serine/threonine-protein kinase 2
- SHARPIN:
-
SHANK-associated RH domain interactor
- SPV:
-
Swine pox virus
- STED:
-
Stimulated emission depletion
- Syk:
-
Spleen tyrosine kinase
- TMS1:
-
Target of methylation-induced gene silencing 1
- TNF:
-
Tumor necrosis factor
- TRAF3:
-
TNF receptor-associated factor 3
- vPOP:
-
Virus-encoded POP
- VSV:
-
Vesicular stomatitis virus
References
Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22:240–273. doi:10.1128/CMR.00046-08
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5. doi:10.1189/jlb.0306164
Razani B, Cheng G (2010) NF-kappaB: much learned, much to learn. Sci Signal. doi:10.1126/scisignal.3138pe29
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411. doi:10.1038/nri3452
Broderick L, De Nardo D, Franklin BS et al (2015) The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol Mech Dis. doi:10.1146/annurev-pathol-012414-040431
Masumoto J, Taniguchi S, Ayukawa K et al (1999) ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem 274:33835–33838
Masumoto J, Taniguchi S, Nakayama J et al (2001) Expression of apoptosis-associated speck-like protein containing a caspase recruitment domain, a pyrin N-terminal homology domain-containing protein, in normal human tissues. J Histochem Cytochem 49:1269–1275. doi:10.1177/002215540104901009
Conway KE, Mcconnell BB, Bowring CE et al (2000) TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res 60:6236–6242
Pelegrin P, Barroso-gutierrez C, Surprenant A (2008) P2X7 receptor differentially couples to distinct release pathways for IL-1 β in mouse macrophage. J Immunol 180:7147–7157. doi:10.4049/jimmunol.180.11.7147
Fernandes-Alnemri T, Wu J, Yu J-W et al (2007) The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ 14:1590–1604. doi:10.1038/sj.cdd.4402194
Martin BN, Wang C, Willette-Brown J et al (2014) IKKα negatively regulates ASC-dependent inflammasome activation. Nat Commun 5:4977. doi:10.1038/ncomms5977
Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C (2009) Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol 182:3173–3182. doi:10.4049/jimmunol.0802367
Wang L, Manji GA, Grenier JM et al (2002) PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J Biol Chem 277:29874–29880. doi:10.1074/jbc.M203915200
Srinivasula SM, Poyet JL, Razmara M et al (2002) The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem 277:21119–21122. doi:10.1074/jbc.C200179200
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Salminen A, Kauppinen A, Hiltunen M, Kaarniranta K (2014) Epigenetic regulation of ASC/TMS1 expression: potential role in apoptosis and inflammasome function. Cell Mol Life Sci 71:1855–1864. doi:10.1007/s00018-013-1524-9
Shiohara M, Taniguchi S, Masumoto J et al (2002) ASC, which is composed of a PYD and a CARD, is up-regulated by inflammation and apoptosis in human neutrophils. Biochem Biophys Res Commun 293:1314–1318. doi:10.1016/S0006-291X(02)00384-4
Stehlik C, Lee SH, Dorfleutner A et al (2003) Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J Immunol 171:6154–6163. doi:10.4049/jimmunol.171.11.6154
Drexler SK, Bonsignore L, Masin M et al (2012) Tissue-specific opposing functions of the inflammasome adaptor ASC in the regulation of epithelial skin carcinogenesis. Proc Natl Acad Sci USA 109:18384–18389. doi:10.1073/pnas.1209171109
Walter A, Schäfer M, Cecconi V et al (2013) Aldara activates TLR7-independent immune defence. Nat Commun 4:1560. doi:10.1038/ncomms2566
Feldmeyer L, Keller M, Niklaus G et al (2007) The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr Biol 17:1140–1145. doi:10.1016/j.cub.2007.05.074
Dombrowski Y, Peric M, Koglin S et al (2011) Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. doi:10.1126/scitranslmed.3002001
Dunn JH, Liu W, Luo Y et al (2012) Dual role of apoptosis-associated speck-like protein containing a CARD (ASC) in tumorigenesis of human melanoma. J Invest Dermatol. doi:10.1038/jid.2012.317
Rathinam VAK, Fitzgerald KA (2016) Review inflammasome complexes: emerging mechanisms and effector functions. Cell 165:792–800. doi:10.1016/j.cell.2016.03.046
Dick MS, Sborgi L, Ru S et al (2016) ASC filament formation serves as a signal amplification mechanism for inflammasome. Nat Commun. doi:10.1038/ncomms11929
Broz P, Dixit VM (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. doi:10.1038/nri.2016.58
Guo H, Callaway JB, Ting JP (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687. doi:10.1038/nm.3893
Kayagaki N, Stowe IB, Lee BL et al (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signaling. Nature. doi:10.1038/nature15541
Shi J, Zhao Y, Wang K et al (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. doi:10.1038/nature15514
Milhavet F, Cuisset L, Hoffman HM et al (2008) The infevers autoinflammatory mutation online registry: update with new genes and functions. Hum Mutat 29:803–808. doi:10.1002/humu.20720
Campbell L, Raheem I, Malemud CJ, Askari AD (2016) The relationship between NALP3 and autoinflammatory syndromes. Int J Mol Sci. doi:10.3390/ijms17050725
Ozen S, Bilginer Y (2014) A clinical guide to autoinflammatory diseases: familial Mediterranean fever and next-of-kin. Nat Rev Rheumatol 10:135–147. doi:10.1038/nrrheum.2013.174
de Alba E (2009) Structure and interdomain dynamics of apoptosis-associated speck-like protein containing a CARD (ASC). J Biol Chem 284:32932–32941. doi:10.1074/jbc.M109.024273
Ravotti F, Sborgi L, Cadalbert R et al (2016) Sequence-specific solid-state NMR assignments of the mouse ASC PYRIN domain in its filament form. Biomol NMR Assign 10:107–115
Park HH, Lo Y-C, Lin S-C et al (2007) The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu Rev Immunol 25:561–586. doi:10.1146/annurev.immunol.25.022106.141656
Huang B, Eberstadt M, Olejniczak ET et al (1996) NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature 384:638–641
Tartaglia LA, Ayres TM, Wong GHW, Goeddel DV (1993) A novel domain within the 55 kd TNF receptor signals cell death. Cell 74:845–853
Liepinsh E, Barbals R, Dahl E et al (2003) The death-domain fold of the ASC PYRIN domain, presenting a basis for PYRIN/PYRIN recognition. J Mol Biol 332:1155–1163. doi:10.1016/j.jmb.2003.07.007
Bae JY, Park HH (2011) Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem 286:39528–39536
Jin T, Perry A, Smith P et al (2013) Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J Biol Chem 288:13225–13235
Pinheiro AS, Proell M, Eibl C et al (2010) Three-dimensional structure of the NLRP7 pyrin domain: insight into pyrin-pyrin-mediated effector domain signaling in innate immunity. J Biol Chem 285:27402–27410
Pinheiro AS, Eibl C, Ekman-Vural Z et al (2011) The NLRP12 pyrin domain: structure, dynamics, and functional insights. J Mol Biol 413:790–803
Hiller S, Kohl A, Fiorito F et al (2003) NMR structure of the apoptosis- and inflammation-related NALP1 pyrin domain. Structure 11:1199–1205
Eibl C, Grigoriu S, Hessenberger M et al (2012) Structural and functional analysis of the NLRP4 pyrin domain. Biochemistry 51:7330–7341
Sborgi L, Ravotti F, Dandey VP et al (2015) Structure and assembly of the mouse ASC inflammasome by combined NMR spectroscopy and cryo-electron microscopy. Proc Natl Acad Sci USA. doi:10.1073/pnas.1507579112
Lu A, Magupalli VG, Ruan J et al (2014) Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156:1193–1206. doi:10.1016/j.cell.2014.02.008
Yuan S, Akey CW (2013) Apoptosome structure, assembly, and procaspase activation. Structure 21:501–515
Richards N, Schaner P, Diaz A et al (2001) Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J Biol Chem 276:39320–39329. doi:10.1074/jbc.M104730200
Cai X, Chen J, Xu H et al (2014) Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156:1207–1222. doi:10.1016/j.cell.2014.01.063
Lu A, Li Y, Yin Q et al (2015) Plasticity in PYD assembly revealed by cryo-EM structure of the PYD filament of AIM2. Cell Discov. doi:10.1038/celldisc.2015.13
Egelman EH, Francis N, DeRosier DJ (1982) F-actin is a helix with a random variable twist. Nature 298:131–135
Chrétien D, Metoz F, Verde F et al (1992) Lattice defects in microtubules: protofilament numbers vary within individual microtubules. J Cell Biol 117:1031–1040
Broz P, von Moltke J, Jones JW et al (2010) Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8:471–483. doi:10.1016/j.chom.2010.11.007
Broz P, Newton K, Lamkanfi M et al (2010) Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207:1745–1755
Schmidt FI, Lu A, Chen JW et al (2016) A single domain antibody fragment that recognizes the adaptor ASC defines the role of ASC domains in inflammasome assembly. J Exp Med. doi:10.1084/jem.20151790
Sahillioglu AC, Sumbul F, Ozoren N, Haliloglu T (2014) Structural and dynamics aspects of ASC speck assembly. Structure 22:1722–1734. doi:10.1016/j.str.2014.09.011
Vajjhala PR, Mirams RE, Hill JM (2012) Multiple binding sites on the pyrin domain of ASC protein allow self-association and interaction with NLRP3 protein. J Biol Chem 287:41732–41743
Stehlik C, Krajewska M, Welsh K et al (2003) The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1 regulation. Biochem J 373:101–113. doi:10.1042/BJ20030304
Vajjhala PR, Kaiser S, Smith SJ et al (2014) Identification of multifaceted binding modes for pyrin and ASC pyrin domains gives insights into pyrin inflammasome assembly. J Biol Chem 289:23504–23519
Masumoto J, Taniguchi S, Sagara J (2001) Pyrin N-terminal homology domain- and caspase recruitment domain-dependent oligomerization of ASC. Biochem Biophys Res Commun 280:652–655. doi:10.1006/bbrc.2000.4190
Proell M, Gerlic M, Mace PD et al (2013) The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J 449:613–621
Masumoto J, Taniguchi S, Nakayama K et al (2001) Murine ortholog of ASC, a CARD-containing protein, self-associates and exhibits restricted distribution in developing mouse embryos. Exp Cell Res 133:128–133. doi:10.1006/excr.2000.5078
Sanders MG, Parsons MJ, Howard AG et al (2015) Single-cell imaging of inflammatory caspase dimerization reveals differential recruitment to inflammasomes. Cell Death Dis 6:e1813. doi:10.1038/cddis.2015.186
Zhang L, Chen S, Ruan J et al (2015) Cryo-EM structure of the activated NAIP2- NLRC4 inflammasome reveals nucleated polymerization. Sci Express 4:12–14. doi:10.1126/science.aac5789
Chen Y-R, Clark AC (2004) Kinetic traps in the folding/unfolding of procaspase-1 CARD domain. Protein Sci 13:2196–2206. doi:10.1110/ps.03521504
Thornberry NA, Bull HG, Calaycay JR et al (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10:417–426. doi:10.1016/S1097-2765(02)00599-3
Lu A, Wu H (2015) Structural mechanisms of inflammasome assembly. FEBS J 5:435–444. doi:10.1111/febs.13133
Hu Z, Zhou Q, Zhang C et al (2015) Structural and biochemical basis for induced self-propagation of NLRC4. Sci Express 4:1–11. doi:10.1126/science.aac5489
Cheng J, Waite AL, Tkaczyk ER et al (2010) Kinetic properties of ASC protein aggregation in epithelial cells. J Cell Physiol 222:738–747. doi:10.1002/jcp.22005
Kueh HY, Mitchison TJ (2009) Structural plasticity in actin and tubulin polymer dynamics. Science 325:960–963
Wu H (2013) Higher-order assemblies in a new paradigm of signal transduction. Cell 153:287–292
Daskalov A, Paoletti M, Ness F, Saupe SJ (2012) Genomic clustering and homology between HET-S and the NWD2 STAND protein in various fungal genomes. PLoS One 7:e34854. doi:10.1371/journal.pone.0034854
Qiao Q, Yang C, Zheng C et al (2013) Structural architecture of the CARMA1/Bcl10/MALT1 signalosome: nucleation-induced filamentous assembly. Mol Cell 51:766–779. doi:10.1016/j.molcel.2013.08.032
Li J, McQuade T, Siemer AB et al (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–350. doi:10.1016/j.cell.2012.06.019
Gaidt MM, Ebert TS, Chauhan D et al (2016) Human monocytes engage an alternative inflammasome pathway. Immunity. doi:10.1016/j.immuni.2016.01.012
Garlanda C, Dinarello CA, Mantovani A (2013) The interleukin-1 family: back to the future. Immunity 39:1003–1018. doi:10.1016/j.immuni.2013.11.010
de Vasconcelos NM, van Opdenbosch N, Lamkanfi M (2016) Inflammasomes as polyvalent cell death platforms. Cell Mol Life Sci 73:2335–2347. doi:10.1007/s00018-016-2204-3
Man SM, Kanneganti T-D (2015) Regulation of inflammasome activation. Immunol Rev 265:6–21
Taniguchi S, Matsushita K, Takeoka M et al (2009) A splice variant of ASC regulates IL-1beta release and aggregates differently from intact ASC. Mediators Inflamm. doi:10.1155/2009/287387
Bryan NB, Dorfleutner A, Kramer SJ et al (2010) Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J Inflamm (Lond) 7:23. doi:10.1186/1476-9255-7-23
Dorfleutner A, Chu L, Stehlik C (2015) Inhibiting the inflammasome: one domain at a time. Immunol Rev 265:205–216
Le HT, Harton JA (2013) Pyrin- and CARD-only proteins as regulators of NLR functions. Front Immunol 4:1–10. doi:10.3389/fimmu.2013.00275
Pawlowski K, Pio F, Chu ZL et al (2001) PAAD—a new protein domain associated with apoptosis, cancer and autoimmune diseases. Trends Biochem Sci 26:85–87. doi:10.1016/S0968-0004(00)01729-1
Natarajan A, Ghose R, Hill JM (2006) Structure and dynamics of ASC2, a pyrin domain-only protein that regulates inflammatory signaling. J Biol Chem 281:31863–31875. doi:10.1074/jbc.M605458200
Espejo F, Patarroyo ME (2006) Determining the 3D structure of human ASC2 protein involved in apoptosis and inflammation. Biochem Biophys Res Commun 340:860–864. doi:10.1016/j.bbrc.2005.12.087
Srimathi T, Robbins SL, Dubas RL et al (2008) Mapping of POP1-binding site on pyrin domain of ASC. J Biol Chem 283:15390–15398
Atianand MK, Harton JA (2011) Uncoupling of Pyrin-only protein 2 (POP2)-mediated dual regulation of NF-κB and the inflammasome. J Biol Chem 286:40536–40547. doi:10.1074/jbc.M111.274290
de Almeida L, Khare S, Misharin AV et al (2015) The PYRIN domain-only protein POP1 inhibits inflammasome assembly and ameliorates inflammatory disease. Immunity 43:264–276
Verhelst K, Verstrepen L, Carpentier I, Beyaert R (2013) IkB kinase e (IKKe): a therapeutic target in inflammation and cancer. Biochem Pharmacol 85:873–880. doi:10.1016/j.bcp.2013.01.007
Dorfleutner A, Bryan NB, Talbott SJ et al (2007) Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect Immun 75:1484–1492
Bedoya F, Sandler LL, Harton JA (2007) Pyrin-only protein 2 modulates NF-κB and disrupts ASC:CLR interactions. J Immunol 178:3837–3845. doi:10.4049/jimmunol.178.6.3837
Atianand MK, Fuchs T, Harton JA (2011) Recent evolution of the NF-κB and inflammasome regulating protein POP2 in primates. BMC Evol Biol 11:56. doi:10.1186/1471-2148-11-56
Khare S, Ratsimandresy RA, de Almeida L et al (2014) The PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat Immunol 15:343–353. doi:10.1038/ni.2829
Porter KA, Duffy EB, Nyland P et al (2014) The CLRX.1/NOD24 (NLRP2P) pseudogene codes a functional negative regulator of NF-κB, pyrin-only protein 4. Genes Immun 15:392–403
Dorfleutner A, Talbott SJ, Bryan NB et al (2007) A Shope Fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes 35:685–694
Johnston JB, Barrett JW, Nazarian SH et al (2005) A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23:587–598. doi:10.1016/j.immuni.2005.10.003
Rahman MM, McFadden G (2011) Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-κB innate response pathways. J Virol 85:12505–12517
Lu A, Li Y, Schmidt FI et al (2016) Molecular basis of caspase-1 polymerization and its inhibition by a new capping mechanism. Nat Struct Mol Biol 23:1–12. doi:10.1038/nsmb.3199
Lamkanfi M, Denecker G, Kalai M et al (2004) INCA, a novel human caspase recruitment domain protein that inhibits interleukin-1β generation. J Biol Chem 279:51729–51738. doi:10.1074/jbc.M407891200
Karasawa T, Kawashima A, Usui F et al (2015) Oligomerized CARD16 promotes caspase-1 assembly and IL-1β processing. FEBS Open Bio 5:348–356. doi:10.1016/j.fob.2015.04.011
Humke EW, Shriver SK, Starovasnik MA et al (2000) ICEBERG: a novel inhibitor of interleukin-1β Generation. Cell 103:99–111. doi:10.1016/S0092-8674(00)00108-2
Lee SH, Stehlik C, Reed JC (2001) COP, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J Biol Chem 276:34495–34500. doi:10.1074/jbc.M101415200
Druilhe A, Srinivasula SM, Razmara M et al (2001) Regulation of IL-1beta generation by pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ 8:649–657. doi:10.1038/sj.cdd.4400881
Kersse K, Lamkanfi M, Bertrand MJM et al (2011) Interaction patches of procaspase-1 caspase recruitment domains (CARDs) are differently involved in procaspase-1 activation and receptor-interacting protein 2 (RIP2)-dependent nuclear factor κB signaling. J Biol Chem 286:35874–35882. doi:10.1074/jbc.M111.242321
Hu H, Sun S-C (2016) Ubiquitin signaling in immune responses. Cell Res 26:457–483. doi:10.1038/cr.2016.40
Cohen P (2014) Immune diseases caused by mutations in kinases and components of the ubiquitin system. Nat Immunol 15:521–529. doi:10.1038/ni.2892
Heaton SM, Borg NA, Dixit VM (2015) Ubiquitin in the activation and attenuation of innate antiviral immunity. J Exp Med 213:1–13. doi:10.1084/jem.20151531
Komander D, Rape M (2012) The ubiquitin code. Annu Rev Biochem 81:203–229. doi:10.1146/annurev-biochem-060310-170328
Hochstrasser M (2009) Origin and function of ubiquitin-like proteins. Nature. doi:10.1038/nature07958
Shimizu Y, Taraborrelli L, Walczak H (2015) Linear ubiquitination in immunity. Immunol Rev 266:190–207. doi:10.1111/imr.12309
Rieser E, Cordier SM, Walczak H (2013) Linear ubiquitination: a newly discovered regulator of cell signalling. Trends Biochem Sci 38:94–102. doi:10.1016/j.tibs.2012.11.007
Rodgers MA, Bowman JW, Fujita H et al (2014) The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J Exp Med 211:1333–1347. doi:10.1084/jem.20132486
Shi C-S, Shenderov K, Huang N-N et al (2012) Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol 13:255–263. doi:10.1038/ni.2215
Matsumoto ML, Dong KC, Yu C et al (2012) Engineering and structural characterization of a linear polyubiquitin-specific antibody. J Mol Biol 418:134–144. doi:10.1016/j.jmb.2011.12.053
Guan K, Wei C, Zheng Z et al (2015) MAVS promotes inflammasome activation by targeting ASC for K63-linked ubiquitination via the E3 ligase TRAF3. J Immunol 194:4880–4890. doi:10.4049/jimmunol.1402851
Saha SK, Pietras EM, He JQ et al (2006) Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J 25:3257–3263. doi:10.1038/sj.emboj.7601220
Park H, Ishihara D, Cox D (2011) Regulation of tyrosine phosphorylation in macrophage phagocytosis and chemotaxis. Arch Biochem Biophys 510:101–111
Clark K (2014) Protein kinase networks that limit TLR signalling. Biochem Soc Trans 42:11–24
Christian F, Smith EL, Carmody RJ (2016) The regulation of NF-κB subunits by phosphorylation. Cells. doi:10.3390/cells5010012
Lin Y-C, Huang D-Y, Wang J-S et al (2015) Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J Leukoc Biol 97:1–11. doi:10.1189/jlb.3HI0814-371RR
Hara H, Tsuchiya K, Kawamura I et al (2013) Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat Immunol 14:1247–1255. doi:10.1038/ni.2749
Gross O, Poeck H, Bscheider M et al (2009) Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. doi:10.1038/nature07965
Zhu F, Xia X, Liu B et al (2007) IKKa shields 14-3-3s, aG2/M cell cycle checkpoint gene, from hypermethylation, preventing its silencing. Mol Cell 27:214–227. doi:10.1016/j.molcel.2007.05.042
Lawrence T, Bebien M, Liu GY et al (2005) IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434:1138–1143. doi:10.1038/nature03491
Balci-Peynircioglu B, Waite AL, Schaner P et al (2008) Expression of ASC in renal tissues of familial mediterranean fever patients with amyloidosis: postulating a role for ASC in AA type amyloid deposition. Exp Biol Med (Maywood) 233:1324–1333. doi:10.3181/0803-RM-106
Franklin BS, Bossaller L, De Nardo D et al (2014) The adaptor ASC has extracellular and “prionoid” activities that propagate inflammation. Nat Immunol. doi:10.1038/ni.2913
Baroja-Mazo A, Martín-Sánchez F, Gomez AI et al (2014) The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 15:1–5. doi:10.1038/ni.2919
Dostert C, Pétrilli V, Van Bruggen R et al (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320:674–677. doi:10.1126/science.1156995
Halle A, Hornung V, Petzold GC et al (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol 9:857–865. doi:10.1038/ni.1636
Hornung V, Bauernfeind F, Halle A et al (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9:847–856. doi:10.1038/ni.1631
Aguzzi A, Nuvolone M, Zhu C (2013) The immunobiology of prion diseases. Nat Rev Immunol 13:888–902. doi:10.1038/nri3553
Holmes BB, Diamond MI (2012) Cellular mechanisms of protein aggregate propagation. Curr Opin Neurol 25:721–726. doi:10.1097/WCO.0b013e32835a3ee0
Serpell LC (2000) Alzheimer’s amyloid fibrils: structure and assembly. Biochim Biophys Acta 1502:16–30. doi:10.1016/S0925-4439(00)00029-6
Greenwald J, Riek R (2010) Biology of amyloid: structure, function, and regulation. Structure 18:1244–1260. doi:10.1016/j.str.2010.08.009
Adamczak S, Dale G, de Rivero Vaccari JP et al (2012) Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg 117:1119–1125. doi:10.3171/2012.9.JNS12815
de Rivero Vaccari JP, Lotocki G, Alonso OF et al (2009) Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab 29:1251–1261. doi:10.1038/jcbfm.2009.46
Sahillioğlu AC, Özören N (2015) Artificial loading of ASC specks with cytosolic antigens. PLoS One 10(8):e0134912. doi:10.1371/journal.pone.0134912
Lelouard H, Gatti E, Cappello F et al (2002) Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature 417:177–182. doi:10.1038/417177a
Knowles TPJ, Vendruscolo M, Dobson CM (2014) The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 15:384–396. doi:10.1038/nrm3810
Morales R, Moreno-Gonzalez I, Soto C (2013) Cross-seeding of misfolded proteins: implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog 9:1–4. doi:10.1371/journal.ppat.1003537
Acknowledgments
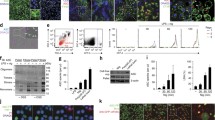
The ASC speck STED image showed in Fig. 2 was recorded with the help of Bjørnar Sporsheim at the Cellular and Molecular Imaging Core Facility (CMIC), Norwegian University of Science and Technology (NTNU). We thank Andrea Stutz for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Hoss and J. F. Rodriguez-Alcazar contributed equally.
Rights and permissions
About this article
Cite this article
Hoss, F., Rodriguez-Alcazar, J.F. & Latz, E. Assembly and regulation of ASC specks. Cell. Mol. Life Sci. 74, 1211–1229 (2017). https://doi.org/10.1007/s00018-016-2396-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-016-2396-6