Abstract
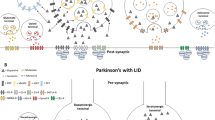
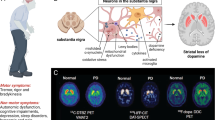
Levodopa-induced dyskinesias (LIDs) occur in the majority of patients with Parkinson’s disease (PD) following years of levodopa treatment. The pathophysiology underlying LIDs in PD is poorly understood, and current treatments generate only minor benefits for the patients. Studies with positron emission tomography (PET) molecular imaging have demonstrated that in advanced PD patients, levodopa administration induces sharp increases in striatal dopamine levels, which correlate with LIDs severity. Fluctuations in striatal dopamine levels could be the result of the attenuated buffering ability in the dopaminergically denervated striatum. Lines of evidence from PET studies indicate that serotonergic terminals could also be responsible for the development of LIDs in PD by aberrantly processing exogenous levodopa and by releasing dopamine in a dysregulated manner from the serotonergic terminals. Additionally, other downstream mechanisms involving glutamatergic, cannabinoid, opioid, cholinergic, adenosinergic, and noradrenergic systems may contribute in the development of LIDs. In this article, we review the findings from preclinical, clinical, and molecular imaging studies, which have contributed to our understanding the pathophysiology of LIDs in PD.


Similar content being viewed by others
Abbreviations
- GIDs:
-
Graft-induced dyskinesias
- LIDs:
-
Levodopa-induced dyskinesias
- PET:
-
Positron emission tomography
- PD:
-
Parkinson’s disease
References
MacDonald BK, Cockerell OC, Sander WAS, Shorvon SD (2000) The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain 123:665–676
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesisa and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Nat Clin Pract Neurol 2:382–392
Nutt JG, Chung KA, Holford NH (2010) Dyskinesia and the antiparkinsonian response always temporally coincide: a retrospective study. Neurology 74:1191–1197
Jankovic J (2005) Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord 20(Suppl 11):S11–S16
Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG (2007) Levodopa-induced dyskinesias. Mov Disord 22:1379–1389
Fahn S (2008) How do you treat motor complications in Parkinson’s disease: medicine, surgery, or both? Ann Neurol 64(Suppl 2):S56–S64
Khlebtovsky A, Rigbi A, Melamed E, Ziv I, Steiner I, Gad A, Djaldetti R (2012) Patient and caregiver perceptions of the social impact of advanced Parkinson’s disease and dyskinesias. J Neural Transm 119:1367–1371
Suh DC, Pahwa R, Mallya U (2012) Treatment patterns and associated costs with Parkinson’s disease levodopa induced dyskinesia. J Neurol Sci 319(1–2):24–31
Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B (2010) Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol 9:1106–1117
Meissner W, Ravenscroft P, Reese R, Harnack D, Morgenstern R, Kupsch A, Klitgaard H, Bioulac B, Gross CE, Bezard E, Boraud T (2006) Increased slow oscillatory activity in substantia nigra pars reticulata triggers abnormal involuntary movements in the 6-OHDA-lesioned rat in the presence of excessive extracellular striatal dopamine. Neurobiol Dis 22:586–598
Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA (2010) L DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 112:1456–1476
Ng KY, Colburn RW, Kopin IJ (1971) Effects of l-dopa on efflux of cerebral monoamines from synaptosomes. Nature 230:331–332
Arai R, Karasawa N, Geffard M, Nagatsu I (1995) l-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a doublelabeling immunofluorescence study. Neurosci Lett 195:195–198
Arai R, Karasawa N, Geffard M, Nagatsu T, Nagatsu I (1994) Immunohistochemical evidence that central serotonin neurons produce dopamine from exogenous l-DOPA in the rat, with reference to the involvement of aromatic l-amino acid decarboxylase. Brain Res 667:295–299
Tanaka H, Kannari K, Maeda T, Tomiyama M, Suda T, Matsunaga M (1999) Role of serotonergic neurons in l-DOPA derived extracellular dopamine in the striatum of 6-OHDA-lesioned rats. NeuroReport 10:631–634
Maeda T, Nagata K, Yoshida Y, Kannari K (2005) Serotonergic hyperinnervation into the dopaminergic denervated striatum compensates for dopamine conversion from exogenously administered l-DOPA. Brain Res 1046:230–233
Carta M, Carlsson T, Kirik D, Björklund A (2007) Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Bibbiani F, Oh JD, Chase TN (2001) Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology 57:18–29
Eskow KL, Gupta V, Alam S, Park JY, Bishop C (2007) The partial 5-HT1A agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improvesl-DOPA efficacy. Pharmacol Biochem Behav 87:306–314
Muñoz A, Qin L, Gardoni F, Marcello E, Qin C, Carlsson T, Kirik D, Di Luca M, Björklund A, Bezard E, Carta M (2008) Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of l-DOPA-induced dyskinesia. Brain 131:3380–3394
Muñoz A, Carlsson T, Tronci E, Kirik, Björklund A, Carta M (2009) Serotonin neuron-dependent and -independent reduction of dyskinesia by 5-HT1A and 5-HT1B receptor agonists in the rat Parkinson model. Exper Neurol 219:298–307
Bishop C, Krolewski D, Eskow K, Barnum C, Dupre K, Deak T, Walker P (2009) Contribution of the striatum to the effects of 5-HT1A receptor stimulation in l-DOPA-treated hemiparkinsonian rats. J Neurosci Res 87:1645–1658
Grégoire L, Samadi P, Grahama J, Bedard P, Bartoszyk G, Di Paolo T (2009) Low doses of sarizotan reduce dyskinesias and maintain antiparkinsonian efficacy of l-Dopa in parkinsonian monkeys. Parkins Rel Disord 15:445–452
Loane C, Politis M (2012) Buspirone: what is it all about? Brain Res 1461:111–118
Bézard E, Muñoz A, Tronci E, Pioli EY, Li Q, Porras G, Björklund A, Carta M (2013) Anti-dyskinetic effect of anpirtoline in animal models of l-DOPA-induced dyskinesia. Neurosci Res 77(4):242–246
Mignon LJ, Wolf WA (2005) 8-Hydroxy-2-(di-n-propylamino)tetralin reduces striatal glutamate in an animal model of Parkinson’s disease. NeuroReport 16:699–703
Dupre KB, Eskow KL, Barnum CJ, Bishop C (2008) Striatal 5-HT(1A) receptor stimulation reduces D1 receptor-induced dyskinesia and improves movement in the hemiparkinsonian rat. Neuropharmacology 55:1321–1328
Gil SJ, Park CH, Lee JE, Minn YK, Koh HC (2011) Positive association between striatal serotonin level and abnormal involuntary movements in chronic l-DOPA-treated hemiparkinsonian rats. Brain Res Bull 84(2):151–156
Conn PJ, Battaglia G, Marino MJ, Nicoletti F (2005) Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci 6:787–798
Finlay C, Duty S (2014) Therapeutic potential of targeting glutamate receptors in Parkinson’s disease. J Neural Transm 121(8):861–880
Dekundy A, Lundblad M, Danysz W, Cenci MA (2007) Modulation of l-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res 179(1):76–89
Picconi B, Pisani A, Centonze D, Battaglia G, Storto M, Nicoletti F, Bernardi G, Calabresi P (2002) Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment. Brain 125:2635–2645
Samadi P, Grégoire L, Morissette M, Calon F, Hadj Tahar A, Bélanger N, Dridi M, Bédard PJ, Di Paolo T (2008) Basal ganglia group II metabotropic glutamate receptors specific binding in non-human primate model of l-Dopa-induced dyskinesias. Neuropharmacology 54:258–268
Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA (2009) Pharmacological modulation of glutamate transmission in a rat model of l-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther 330(1):227–235
Samadi P, Grégoire L, Morissette M, Calon F, Hadj Tahar A, Dridi M, Belanger N, Meltzer LT, Bédard PJ, Di Paolo T (2008) mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiol Aging 29:1040–1051
Ouattara B, Grégoire L, Morissette M, Gasparini F, Vranesic I, Bilbe G, Johns DR, Rajput A, Hornykiewicz O, Rajput AH, Gomez-Mancilla B, Di Paolo T (2011) Metabotropic glutamate receptor type 5 in levodopa-induced motor complications. Neurobiol Aging 32:1286–1295
Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W (2006) Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res Bull 69:318–326
Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA (2007) Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson’s disease. J Neurochem 101:483–497
Rylander D, Iderberg H, Li Q, Dekundy A, Zhang J, Li H, Baishen R, Danysz W, Bezard E, Cenci MA (2010) A mGluR5 antagonist under clinical development improves l-DOPA-induced dyskinesia in parkinsonian rats and monkeys. Neurobiol Dis 39:352–361
Dekundy A, Gravius A, Hechenberger M, Pietraszek M, Nagel J, Tober C, van der Elst M, Mela F, Parsons CG, Danysz W (2011) Pharmacological characterization of MRZ-8676, a novel negative allosteric modulator of subtype 5 metabotropic glutamate receptors (mGluR5): focus on L: -DOPA-induced dyskinesia. J Neural Transm 118:1703–1716
Grégoire L, Morin N, Ouattara B, Gasparini F, Bilbe G, Johns D, Vranesic I, Sahasranaman S, Gomez-Mancilla B, Di Paolo T (2011) The acute antiparkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in l-Dopa-treated parkinsonian monkeys. Parkinsonism Relat Disord 17:270–276
Morelli M, Carta AR, Jenner P (2009) Adenosine A2A receptors and Parkinson’s disease. Handb Exp Pharmacol 193:589–615
Calon F, Dridi M, Hornykiewicz O, Bedard PJ, Rajput AH, Di Paolo T (2004) Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain 127:1075–1084
Morissette M, Dridi M, Calon F, Hadj Tahar A, Meltzer LT, Bédard PJ, Di Paolo T (2006) Prevention of dyskinesia by an NMDA receptor antagonist in MPTP monkeys: effect on adenosine A2A receptors. Synapse 60:239–250
Zeng BY, Pearce RK, MacKenzie GM, Jenner P (2000) Alterations in preproenkephalin and adenosine-2a receptor mRNA, but not preprotachykinin mRNA correlate with occurrence of dyskinesia in normal monkeys chronically treated with l-DOPA. Eur J Neurosci 12:1096–1104
Bibbiani F, Oh JD, Petzer JP, Castagnoli N Jr, Chen JF, Schwarzschild MA, Chase TN (2003) A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease. Exp Neurol 184:285–294
Phelps ME (2000) Positron emission tomography provides molecular imaging of biological processes. PNAS 97:9226–9233
Loane C, Politis M (2011) Positron emission tomography neuroimaging in Parkinson’s disease. Am J Transl Res. 3(4):323–341
Politis M, Piccini P (2012) Positron emission tomography imaging in neurological disorders. J Neurol 259:1769–1780
Politis M, Niccolini F (2015) Serotonin in Parkinson’s disease. Behav Brain Res 277:136–145
Niccolini F, Su P, Politis M (2014) Dopamine receptor mapping with PET imaging in Parkinson’s disease. J Neurol 261:2251–2263
Niccolini F, Loane C, Politis M (2014) Dyskinesias in Parkinson’s disease: views from positron emission tomography studies. Eur J Neurol 21(694–9):e39–e43
de la Fuente-Fernandez R, Lu JQ, Sossi V, Jivan S, Schulzer M, Holden JE, Lee CS, Ruth TJ, Calne DB, Stoessl AJ (2001) Biochemical variations in the synaptic levels of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol 49:298–303
de la Fuente-Fernández R, Lim AS, Sossi V, Holden JE, Calne DB, Ruth TJ, Stoessl AJ (2001) Apomorphine-induced changes in synaptic dopamine levels: positron emission tomography evidence for presynaptic inhibition. J Cereb Blood Flow Metab 21:1151–1159
de la Fuente-Fernandez R, Sossi V, Huang Z, Furtado S, Lu QR, Calne DB, Ruth TJ, Stoessl AJ (2004) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain 127:2747–2754
Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, Piccini P (2006) Clinical correlates of levodopa induced dopamine release in Parkinson’s disease: a PET study. Neurology 67:1612–1617
Politis M, Wu K, Loane C, Brooks DJ, Kiferle L, Turkheimer FE, Bain P, Molloy S, Piccini P (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Invest 124:1340–1349
Goetz CG, Laska E, Hicking C, Damier P, Müller T, Nutt J, Warren Olanow C, Rascol O, Russ H (2008) Placebo influences on dyskinesia in Parkinson’s disease. Mov Disord 23:700–707
Müller T, Olanow CW, Nutt J (2006) The PADDY-2 study: the evaluation of sarizotan for treatment-associated dyskinesia in Parkinson’s disease patients. Mov Disord 21(Suppl 15):S591
Goetz CG, Damier P, Hicking C, Laska E, Müller T, Olanow CW, Rascol O, Russ H (2007) Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord 22:179–186
Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, Crabb L, Pavese N, Oertel WH, Quinn N, Brooks DJ, Lindvall O (2002) Dyskinesias following neural transplantation in Parkinson’s disease. Nat Neurosci 5:627–628
Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D (2002) Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol 52:628–634
Olanow CW, Gracies JM, Goetz CG, Stoessl AJ, Freeman T, Kordower JH, Godbold J, Obeso JA (2009) Clinical pattern and risk factors for dyskinesias following fetal nigral transplantation in Parkinson’s disease: a double blind video-based analysis. Mov Disord 24:336–343
Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Rehncrona S, Bjorklund A, Lindvall O, Piccini P (2010) Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci Transl Med 2:38–46
Politis M (2010) Dyskinesias after neural transplantation in Parkinson’s disease: what do we know and what is next? BMC Med 8:80
Politis M, Oertel WH, Wu K, Quinn NP, Pogarell O, Brooks DJ, Bjorklund A, Lindvall O, Piccini P (2011) Graft-induced dyskinesias in Parkinson’s disease: high striatal serotonin/dopamine transporter ratio. Mov Disord 26:1997–2003
Politis M, Wu K, Loane C, Quinn NP, Brooks DJ, Oertel WH, Björklund A, Lindvall O, Piccini P (2012) Serotonin neuron loss and nonmotor symptoms continue in Parkinson’s patients treated with dopamine grafts. Sci Transl Med 4:128–141
Politis M, Piccini P (2010) Brain imaging after neural transplantation. Prog Brain Res 184:193–203
Politis M, Piccini P (2012) In vivo imaging of the integration and function of nigral grafts in clinical trials. Prog Brain Res 2000:199–220
Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Björklund A, Lindvall O, Limousin P, Quinn N, Foltynie T (2014) Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol 71:83–87
Loane C, Politis M (2012) Buspirone: what is it all about? Brain Res 21(1461):111–118
Duty S (2012) Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs 26:1017–1032
Ahmed I, Bose S, Pavese N, Ramlackhansingh A, Turkheimer F, Hotton G, Hammers A, Brooks DJ (2011) Glutamate NMDA receptor dysregulation in Parkinson’s disease with dyskinesias. Brain 134:979–986
Crosby NJ, Deane K, Clarke CE (2003) Amantadine for dyskinesia in Parkinson’s disease. Cochrane Database Syst Rev 2:CD003467
Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN (1998) Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 50:1323–1326
Luginger E, Wenning GK, Bösch S, Poewe W (2000) Beneficial effects of amantadine on l-dopa-induced dyskinesias in Parkinson’s disease. Mov Disord 15:873–878
Snow BJ, Macdonald L, Mcauley D, Wallis W (2000) The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol 23:82–85
Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M (2004) Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatr 75:141–143
Berg D, Godau J, Trenkwalder C, Eggert K, Csoti I, Storch A, Huber H, Morelli-Canelo M, Stamelou M, Ries V, Wolz M, Schneider C, Di Paolo T, Gasparini F, Hariry S, Vandemeulebroecke M, Abi-Saab W, Cooke K, Johns D, Gomez-Mancilla B (2011) AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord 26:1243–1250
Stocchi F, Rascol O, Destee A, Hattori N, Hauser RA, Lang AE, Poewe W, Stacy M, Tolosa E, Gao H, Nagel J, Merschhemke M, Graf A, Kenney C, Trenkwalder C (2013) AFQ056 in Parkinson patients with l-DOPA-induced dyskinesia: 13-week dose-finding study. Mov Disord 28:1838–1846
Mishina M, Ishiwata K, Naganawa M, Kimura Y, Kitamura S, Suzuki M, Hashimoto M, Ishibashi K, Oda K, Sakata M, Hamamoto M, Kobayashi S, Katayama Y, Ishii K (2011) Adenosine A2A receptors measured with [11C]TMSX PET in the striata of Parkinson’s disease patients. PLoS One 6:e17338
Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ (2011) Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology 76:1811–1816
Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M, Japanese Istradefylline Study Group (2010) Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord 25:1437–1443
Pourcher E, Fernandez HH, Stacy M, Mori A, Ballerini R, Chaikin P (2012) Istradefylline for Parkinson’s disease patients experiencing motor fluctuations: results of the KW-6002-US-018 study. Parkinsonism Relat Disord 18:178–184
Hauser RA, Cantillon M, Pourcher E, Micheli F, Mok V, Onofrj M, Huyck S, Wolski K (2011) Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol 10:221–229
Samadi P, Bedard PJ, Rouillard C (2006) Opioids and motor complications in Parkinson’s disease. Trends Pharmacol Sci 27:512–517
Piccini P, Ra Weeks, Brooks DJ (1997) Alterations in opioid receptor binding in Parkinson’s disease patients with levodopa-induced dyskinesias. Ann Neurol 42:720–726
Sadzot B, Price JC, Mayberg HS, Douglass KH, Dannals RF, Lever JR, Ravert HT, Wilson AA, Wagner HN Jr, Feldman MA (1992) Quantification of human opiate receptor concentration and affinity using high and low specific activity [11C]diprenorphine and positron emission tomography. J Cereb Blood Flow Metab 12:885
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991) Characterization and localization of cannabinoid receptors in the rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11:563–583
Mailleux P, Vanderhaeghen JJ (1992) Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neurosci Lett 148:173–176
Glass M, Dragunow M, Faull RLM (1997) Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299–318
Martín AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, de Fonseca FR, Moratalla R (2008) Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology 33:1667–1679
van Laere K, Casteels C, Lunskens S, Goffin K, Grachev ID, Bormans G, Vandenberghe W (2012) Regional changes in type 1 cannabinoid receptor availability in Parkinson’s disease in vivo. Neurobiol Aging 33:620
Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM (2001) Cannabinoids reduce levodopa-induced dyskinesia in Parkinson’s disease: a pilot study. Neurology 57:2108–2111
Venderová K, Růzicka E, Vorísek V, Visnovský P (2004) Survey on cannabis use in Parkinson’s disease: subjective improvement of motor symptoms. Mov Disord 19:1102–1106
Zhou FM, Wilson CJ, Dani JA (2002) Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol 53:590–605
Kitabatake Y, Hikida T, Watanabe D, Pastan I, Nakanishi S (2003) Impairment of reward-related learning by cholinergic cell ablation in the striatum. Proc Natl Acad Sci USA 100:7965–7970
Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, Kreitzer AC (2014) Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron 82(1):63–70
Quik M, Cox H, Parameswaran N, O’Leary K, Langston JW, Di Monte D (2007) Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol 62:588–596
Bordia T, Campos C, Huang L, Quik M (2008) Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (l-DOPA)-induced dyskinesias in a rat model of Parkinson’s disease. J Pharmacol Exp Ther 327:239–247
Huang L, Grady SR, Quik M (2011) Nicotine reduces l-DOPA-induced dyskinesias by acting at beta2 nicotinic receptors. J Pharmacol Exp Ther 338:932–941
Quik M, Mallela A, Ly J, Zhang D (2013) Nicotine reduces established levodopa-induced dyskinesias in a monkey model of Parkinson’s disease. Mov Disord 28:1398–1406
Zhang D, Mallela A, Sohn D, Carroll FI, Bencherif M, Letchworth S, Quik M (2013) Nicotinic receptor agonists reduce l-DOPA-induced dyskinesias in a monkey model of Parkinson’s disease. J Pharmacol Exp Ther 347:225–234
Rinne JO, Myllykyla T, Lonnberg P, Marjamaki P (1991) A postmortem study of brain nicotinic receptors in Parkinson’s and Alzheimer’s disease. Brain Res 547:167–170
Quik M, Bordia T, Forno L, McIntosh JM (2004) Loss of alpha-conotoxinMII- and A85380-sensitive nicotinic receptors in Parkinson’s disease striatum. J Neurochem 88:668–679
Schmaljohann J, Gundisch D, Minnerop M, Bucerius J, Joe A, Reinhardt M, Guhlke S, Biersack HJ, Wullner U (2006) In vitro evaluation of nicotinic acetylcholine receptors with 2-[18F]F-A85380 in Parkinson’s disease. Nucl Med Biol 33:305–309
Kas A, Bottlaender M, Gallezot JD, Vidailhet M, Villafane G, Grégoire MC, Coulon C, Valette H, Dolle F, Ribeiro MJ, Hantraye P, Remy P (2009) Decrease of nicotinic receptors in the nigrostriatal system in Parkinson’s disease. J Cereb Blood Flow 29:1601–1608
German DC, Manaye KF, White CL III, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM (1992) Disease-specific patterns of locus coeruleus cell loss. Ann Neurol 32:667–676
Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60:337–341
Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC (1991) Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson’s disease. Neuroscience 41:507–523
Shin E, Rogers JT, Devoto P, Björklund A, Carta M (2014) Noradrenaline neuron degeneration contributes to motor impairments and development of l-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Exp Neurol 257:25–38
Arai A, Tomiyama M, Kannari K, Kimura T, Suzuki C, Watanabe M, Kawarabayashi T, Shen H, Shoji M (2008) Reuptake of l-DOPA-derived extracellular DA in the striatum of a rodent model of Parkinson’s disease via norepinephrine transporter. Synapse 62:632–635
Gomez-Mancilla B, Bédard PJ (1993) Effect of nondopaminergic drugs on l-dopa-induced dyskinesias in MPTP-treated monkeys. Clin Neuropharmacol 16:418–427
Buck K, Ferger B (2010) The selective alpha1 adrenoceptor antagonist HEAT reduces l-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Synapse 64:117–126
Lindenbach D, Ostock CY, Eskow Jaunarajs KL, Dupre KB, Barnum CJ, Bhide N, Bishop C (2011) Behavioral and cellular modulation of l-DOPA-induced dyskinesia by beta-adrenoceptor blockade in the 6-hydroxydopamine-lesioned rat. J Pharmacol Exp Ther 337:755–765
Carpentier AF, Bonnet AM, Vidailhet M, Agid Y (1996) Improvement of levodopa-induced dyskinesia by propranolol in Parkinson’s disease. Neurology 46:1548–1551
Buck K, Voehringer P, Ferger B (2010) The alpha(2) adrenoceptor antagonist idazoxan alleviates l-DOPA induced dyskinesia by reduction of striatal dopamine levels: an in vivo microdialysis study in 6-hydroxydopamine-lesioned rats. J Neurochem 112:444–452
Fox SH, Henry B, Hill MP, Peggs D, Crossman AR, Brotchie JM (2001) Neural mechanisms underlying peak-dose dyskinesia induced by levodopa and apomorphine are distinct: evidence from the effects of the alpha(2) adrenoceptor antagonist idazoxan. Mov Disord 16:642–650
Grondin R, Hadj Tahar A, Doan VD, Ladure P, Bedard PJ (2000) Noradrenoceptor antagonism with idazoxan improves l-dopa-induced dyskinesias in MPTP monkeys. Naunyn Schmiedebergs Arch Pharmacol 361:181–186
Savola JM, Hill M, Engstrom M, Merivuori H, Wurster S, McGuire SG (2003) Fipamezole (JP-1730) is a potent alpha2 adrenergic receptor antagonist that reduces levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson’s disease. Mov Disord 18:872–883
Rascol O, Arnulf I, Peyro-Saint Paul H, Brefel-Courbon C, Vidailhet M, Thalamas C (2001) Idazoxan, an alpha-2 antagonist, and l-DOPA-induced dyskinesias in patients with Parkinson’s disease. Mov Disord 16:708–713
Lewitt PA, Hauser RA, Lu M, Nicholas AP, Weiner W, Coppard N, Leinonen M, Savola JM (2012) Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study). Neurology 79:163–169
Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P (1992) Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N, N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA 89:8155–8159
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151
Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC (1997) Induction of dopamine D3 receptor expression as a mechanism of behavioural sensitization to levodopa. Proc Natl Acad Sci USA 94:3363–3367
Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P (2003) Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med 9:762–767
van Kampen JM, Stoessl AJ (2003) Effects of oligonucleotide antisense to dopamineD3 receptor mRNA in a rodent model of behavioural sensitization to levodopa. Neuroscience 116:307–314
Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Håkansson K, Bioulac BH, Gross CE, Sokoloff P, Fisone G, Gurevich EV, Bloch B, Bezard E (2005) Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism Relat Disord 11(Suppl. 1):S25–S29
Visanji NP, Fox SH, Johnston T, Reyes G, Millan MJ, Brotchie JM (2009) DopamineD3 receptor stimulation underlies the development of l-DOPA-induced dysk-inesia in animal models of Parkinson’s disease. Neurobiol Dis 35:184–192
Cote SR, Chitravanshi VC, Bleickardt C, Sapru HN, Kuzhikandathil EV (2014) Overexpression of the dopamine D3 receptor in the rat dorsal striatum induces dyskinetic behaviors. Behav Brain Res 263:46–50
Kumar R, Riddle L, Griffin SA, Grundt P, Newman AH, Luedtke RR (2009) Evaluation of the D3 dopamine receptor selective antagonist PG01037 on l-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology 56:944–955
Kumar R, Riddle LR, Griffin SA, Chu W, Vangveravong S, Neisewander J, Mach RH, Luedtke RR (2009) Evaluation of D2 and D3 dopamine receptor selective compounds on l-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology 56:956–969
Mela F, Millan MJ, Brocco M, Morari M (2010) The selective D(3) receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect l-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats. Neuropharmacology 58:528–536
Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, Diwan M, Wilson AA, McCormick P, Gentile G, Gunn RN, Laruelle MA (2009) In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: studies in non-human primates and transgenic mice. Synapse 63:782–793
Boileau I, Guttman M, Rusjan P, Adams JR, Houle S, Tong J, Hornykiewicz O, Furukawa Y, Wilson AA, Kapur S, Kish SJ (2009) Decreased binding of the D3 dopamine receptor-preferring ligand [11C]-(+)-PHNO in drug-naive Parkinson’s disease. Brain 132:1366–1375
Nishi A, Kuroiwa M, Shuto T (2011) Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Front Neuroanat 5:43
Menniti FS, Faraci WS, Schmidt CJ (2006) Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov 5:660–670
Nishi A, Kuroiwa M, Miller DB, O’Callaghan JP, Bateup HS, Shuto T, Sotogaku N, Fukuda T, Heintz N, Greengard P, Snyder GL (2008) Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci 28:10460–10471
Greengard P, Allen PB, Nairn AC (1999) Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23:435–447
Girault JA (2012) Integrating neurotransmission in striatal medium spiny neurons. Adv Exp Med Biol 970:407–429
Hossain MA, Weiner N (1993) Dopaminergic functional supersensitivity: effects of chronic l-dopa and carbidopa treatment in an animal model of Parkinson’s disease. J Pharmacol Exp Ther 267:1105–1111
Tenn CC, Niles LP (1997) Sensitization of G protein-coupled benzodiazepine receptors in the striatum of 6-hydroxydopamine-lesioned rats. J Neurochem 69:1920–1926
Giorgi M, D’Angelo V, Esposito Z, Nuccetelli V, Sorge R, Martorana A, Stefani A, Bernardi G, Sancesario G (2008) Lowered cAMP and cGMP signalling in the brain during levodopa-induced dyskinesias in hemiparkinsonian rats: new aspects in the pathogenetic mechanisms. Eur J Neurosci 28:941–950
Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P (2003) Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci 6:501–506
Picconi B, Paillé V, Ghiglieri V, Bagetta V, Barone I, Lindgren HS, Bernardi G, Angela Cenci M, Calabresi P (2008) l-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol Dis 29:327–335
Picconi B, Bagetta V, Ghiglieri V, Paillè V, Di Filippo M, Pendolino V, Tozzi A, Giampà C, Fusco FR, Sgobio C, Calabresi P (2011) Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain 134:375–387
Cenci MA (2010) Pathophysiology of l-DOPA-induced dyskinesia in parkinson’s disease. In: Dunnett S, Björklund A, Iversen L, Iversen S (eds) Dopamine Handbook. Oxford University Press, New York, pp 434–444
Acknowledgments
Flavia Niccolini research was supported from Parkinson’s UK. Lorenzo Rocchi research is supported from the Lily and Edmond J. Safra Foundation. Marios Politis research was supported by Parkinson’s UK, Lily and Edmond J. Safra Foundation, Imanova ltd, Michael J Fox Foundation (MJFF), and NIHR BRC.
Conflict of interest
The authors declare no financial or other conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niccolini, F., Rocchi, L. & Politis, M. Molecular imaging of levodopa-induced dyskinesias. Cell. Mol. Life Sci. 72, 2107–2117 (2015). https://doi.org/10.1007/s00018-015-1854-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1854-x