Abstract
In cells, the levels of sterol vary greatly among organelles. This uneven distribution depends largely on non-vesicular routes of transfer, which are mediated by soluble carriers called lipid-transfer proteins (LTPs). These proteins have a domain with a hydrophobic cavity that accommodates one sterol molecule. However, a demonstration of their role in sterol transport in cells remains difficult. Numerous LTPs also contain membrane-binding elements, but it is not clear how these LTPs couple their ability to target organelles with lipid transport activity. This issue appears critical, since many sterol transporters are thought to act at contact sites between two membrane-bound compartments. Here, we emphasize that biochemical and structural studies provide precious insights into the mode of action of sterol-binding proteins. Recent studies on START, Osh/ORP and NPC proteins suggest models on how these proteins could transport sterol between organelles and, thereby, influence cellular functions.




Similar content being viewed by others
Abbreviations
- DHE:
-
Dehydroergosterol
- ER:
-
Endoplasmic reticulum
- ERC:
-
Endosomal recycling compartment
- FFAT:
-
Two phenylalanines in an acidic tract
- LTP:
-
Lipid tranfer protein
- LBPA:
-
Lysobisphosphatidic acid
- LE/LY :
-
Late endosome/lysosome
- MCD:
-
Methyl-β-cyclodextrin
- NPC:
-
Niemann-Pick C
- ORP:
-
Oxysterol-binding protein-related protein
- ORD:
-
OSBP-related domain
- OSBP:
-
Oxysterol-binding protein
- Osh:
-
Oxysterol-binding homology protein
- PH:
-
Pleckstrin homology
- PI:
-
Phosphatidylinositol
- PI(4)P:
-
Phosphatidylinositol 4-phosphate
- PIP:
-
Phosphatidylinositol phosphate
- PM:
-
Plasma membrane
- SM:
-
Sphingomyelin
- StAR:
-
Steroidogenic acute regulatory protein
- START:
-
StAR-related lipid transfer
- TGN:
-
trans-Golgi network
References
Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS (2008) Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab 8:512–521
Maxfield FR, Menon AK (2006) Intracellular sterol transport and distribution. Curr Opin Cell Biol 18:379–385
Liscum L, Munn NJ (1999) Intracellular cholesterol transport. Biochim Biophys Acta 1438:19–37
Blanchette-Mackie EJ (2000) Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim Biophys Acta 1486:171–183
Sullivan DP, Ohvo-Rekila H, Baumann NA, Beh CT, Menon AK (2006) Sterol trafficking between the endoplasmic reticulum and plasma membrane in yeast. Biochem Soc Trans 34:356–358
van Meer G, Voelker DR, Feigenson GW (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124
Maxfield FR, van Meer G (2010) Cholesterol, the central lipid of mammalian cells. Curr Opin Cell Biol 22:422–429
Mesmin B, Maxfield FR (2009) Intracellular sterol dynamics. Biochim Biophys Acta 1791:636–645
Lange Y, Steck TL (2008) Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog Lipid Res 47:319–332
Phillips MC, Johnson WJ, Rothblat GH (1987) Mechanisms and consequences of cellular cholesterol exchange and transfer. Biochim Biophys Acta 906:223–276
Prinz WA (2010) Lipid trafficking sans vesicles: where, why, how? Cell 143:870–874
Baumann NA, Sullivan DP, Ohvo-Rekila H, Simonot C, Pottekat A, Klaassen Z, Beh CT, Menon AK (2005) Transport of newly synthesized sterol to the sterol-enriched plasma membrane occurs via nonvesicular equilibration. Biochemistry 44:5816–5826
Ohvo-Rekila H, Ramstedt B, Leppimaki P, Slotte JP (2002) Cholesterol interactions with phospholipids in membranes. Prog Lipid Res 41:66–97
Leventis R, Silvius JR (2001) Use of cyclodextrins to monitor transbilayer movement and differential lipid affinities of cholesterol. Biophys J 81:2257–2267
Radhakrishnan A, McConnell HM (1999) Condensed complexes of cholesterol and phospholipids. Biophys J 77:1507–1517
Schneiter R, Brugger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G, Paltauf F, Wieland FT, Kohlwein SD (1999) Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J Cell Biol 146:741–754
Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G (1991) Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 173:2026–2034
Klemm RW, Ejsing CS, Surma MA, Kaiser HJ, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K (2009) Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol 185:601–612
Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G (2001) A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur J Biochem 268:2351–2361
Soccio RE, Breslow JL (2003) StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J Biol Chem 278:22183–22186
Miller WL (2007) Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta 1771:663–676
Alpy F, Tomasetto C (2005) Give lipids a START: the StAR-related lipid transfer (START) domain in mammals. J Cell Sci 118:2791–2801
Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M (2003) Molecular machinery for non-vesicular trafficking of ceramide. Nature 426:803–809
Soccio RE, Adams RM, Romanowski MJ, Sehayek E, Burley SK, Breslow JL (2002) The cholesterol-regulated StarD4 gene encodes a StAR-related lipid transfer protein with two closely related homologues, StarD5 and StarD6. Proc Natl Acad Sci USA 99:6943–6948
Soccio RE, Adams RM, Maxwell KN, Breslow JL (2005) Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. Activation of StarD4 by sterol regulatory element-binding protein-2 and StarD5 by endoplasmic reticulum stress. J Biol Chem 280:19410–19418
Tabas I (2010) The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res 107:839–850
Rodriguez-Agudo D, Ren S, Hylemon PB, Montanez R, Redford K, Natarajan R, Medina MA, Gil G, Pandak WM (2006) Localization of StarD5 cholesterol binding protein. J Lipid Res 47:1168–1175
Brown MS, Goldstein JL (2009) Cholesterol feedback: from Schoenheimer’s bottle to Scap’s MELADL. J Lipid Res 50(Suppl):S15–S27
Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL (2003) Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA 100:12027–12032
Rodriguez-Agudo D, Calderon-Dominguez M, Ren S, Marques D, Redford K, Medina-Torres MA, Hylemon P, Gil G, Pandak WM (2011) Subcellular localization and regulation of StarD4 protein in macrophages and fibroblasts. Biochim Biophys Acta 1811:597–606
Rodriguez-Agudo D, Ren S, Wong E, Marques D, Redford K, Gil G, Hylemon P, Pandak WM (2008) Intracellular cholesterol transporter StarD4 binds free cholesterol and increases cholesteryl ester formation. J Lipid Res 49:1409–1419
Mesmin B, Pipalia NH, Lund FW, Ramlall TF, Sokolov A, Eliezer D, Maxfield FR (2011) STARD4 abundance regulates sterol transport and sensing. Mol Biol Cell 22:4004–4015
Riegelhaupt JJ, Waase MP, Garbarino J, Cruz DE, Breslow JL (2010) Targeted disruption of steroidogenic acute regulatory protein D4 leads to modest weight reduction and minor alterations in lipid metabolism. J Lipid Res 51:1134–1143
Thorsell AG, Lee WH, Persson C, Siponen MI, Nilsson M, Busam RD, Kotenyova T, Schuler H, Lehtio L (2011) Comparative structural analysis of lipid binding START domains. PLoS ONE 6:e19521
Romanowski MJ, Soccio RE, Breslow JL, Burley SK (2002) Crystal structure of the Mus musculus cholesterol-regulated START protein 4 (StarD4) containing a StAR-related lipid transfer domain. Proc Natl Acad Sci USA 99:6949–6954
Murcia M, Faraldo-Gomez JD, Maxfield FR, Roux B (2006) Modeling the structure of the StART domains of MLN64 and StAR proteins in complex with cholesterol. J Lipid Res 47:2614–2630
Tsujishita Y, Hurley JH (2000) Structure and lipid transport mechanism of a StAR-related domain. Nat Struct Biol 7:408–414
Lavigne P, Najmanivich R, Lehoux JG (2010) Mammalian StAR-related lipid transfer (START) domains with specificity for cholesterol: structural conservation and mechanism of reversible binding. Subcell Biochem 51:425–437
Bose HS, Whittal RM, Baldwin MA, Miller WL (1999) The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc Natl Acad Sci USA 96:7250–7255
Baker BY, Yaworsky DC, Miller WL (2005) A pH-dependent molten globule transition is required for activity of the steroidogenic acute regulatory protein, StAR. J Biol Chem 280:41753–41760
Kudo N, Kumagai K, Tomishige N, Yamaji T, Wakatsuki S, Nishijima M, Hanada K, Kato R (2008) Structural basis for specific lipid recognition by CERT responsible for nonvesicular trafficking of ceramide. Proc Natl Acad Sci USA 105:488–493
van Tiel CM, Schouten A, Snoek GT, Gros P, Wirtz KW (2002) The structure of phosphatidylinositol transfer protein alpha reveals sites for phospholipid binding and membrane association with major implications for its function. FEBS Lett 531:69–73
Leventis PA, Grinstein S (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys 39:407–427
Clark BJ, Wells J, King SR, Stocco DM (1994) The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322
Bose H, Lingappa VR, Miller WL (2002) Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 417:87–91
Miller WL, Bose HS (2011) Early steps in steroidogenesis: intracellular cholesterol trafficking. J Lipid Res 52:2111–2135
Clark BJ (2012) The mammalian START domain protein family in lipid transport in health and disease. J Endocrinol 212:257–275
Brown MS, Goldstein JL (1979) Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci USA 76:3330–3337
Pentchev PG (2004) Niemann–Pick C research from mouse to gene. Biochim Biophys Acta 1685:3–7
Pipalia NH, Hao M, Mukherjee S, Maxfield FR (2007) Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann–Pick type C1 defect. Traffic 8:130–141
Maxfield FR, Tabas I (2005) Role of cholesterol and lipid organization in disease. Nature 438:612–621
Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE (2009) Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137:1213–1224
Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS (2008) Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem 283:1064–1075
Friedland N, Liou HL, Lobel P, Stock AM (2003) Structure of a cholesterol-binding protein deficient in Niemann–Pick type C2 disease. Proc Natl Acad Sci USA 100:2512–2517
Xu S, Benoff B, Liou HL, Lobel P, Stock AM (2007) Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann–Pick type C2 disease. J Biol Chem 282:23525–23531
Xu Z, Farver W, Kodukula S, Storch J (2008) Regulation of sterol transport between membranes and NPC2. Biochemistry 47:11134–11143
McCauliff LA, Xu Z, Storch J (2011) Sterol transfer between cyclodextrin and membranes: similar but not identical mechanism to NPC2-mediated cholesterol transfer. Biochemistry 50:7341–7349
Storch J, Xu Z (2009) Niemann–Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim Biophys Acta 1791:671–678
Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL (2008) NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci USA 105:15287–15292
Wang ML, Motamed M, Infante RE, Abi-Mosleh L, Kwon HJ, Brown MS, Goldstein JL (2010) Identification of surface residues on Niemann–Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab 12:166–173
Deffieu MS, Pfeffer SR (2011) Niemann–Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc Natl Acad Sci USA 108:18932–18936
Davies JP, Ioannou YA (2000) Topological analysis of Niemann–Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem 275:24367–24374
Millard EE, Gale SE, Dudley N, Zhang J, Schaffer JE, Ory DS (2005) The sterol-sensing domain of the Niemann–Pick C1 (NPC1) protein regulates trafficking of low density lipoprotein cholesterol. J Biol Chem 280:28581–28590
Alpy F, Stoeckel ME, Dierich A, Escola JM, Wendling C, Chenard MP, Vanier MT, Gruenberg J, Tomasetto C, Rio MC (2001) The steroidogenic acute regulatory protein homolog MLN64, a late endosomal cholesterol-binding protein. J Biol Chem 276:4261–4269
Charman M, Kennedy BE, Osborne N, Karten B (2010) MLN64 mediates egress of cholesterol from endosomes to mitochondria in the absence of functional Niemann–Pick Type C1 protein. J Lipid Res 51:1023–1034
Watari H, Arakane F, Moog-Lutz C, Kallen CB, Tomasetto C, Gerton GL, Rio MC, Baker ME, Strauss JF 3rd (1997) MLN64 contains a domain with homology to the steroidogenic acute regulatory protein (StAR) that stimulates steroidogenesis. Proc Natl Acad Sci USA 94:8462–8467
Kishida T, Kostetskii I, Zhang Z, Martinez F, Liu P, Walkley SU, Dwyer NK, Blanchette-Mackie EJ, Radice GL, Strauss JF 3rd (2004) Targeted mutation of the MLN64 START domain causes only modest alterations in cellular sterol metabolism. J Biol Chem 279:19276–19285
Gill S, Chow R, Brown AJ (2008) Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res 47:391–404
Borthwick F, Allen AM, Taylor JM, Graham A (2010) Overexpression of STARD3 in human monocyte/macrophages induces an anti-atherogenic lipid phenotype. Clin Sci (Lond) 119:265–272
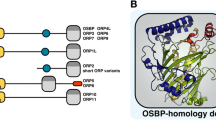
Jaworski CJ, Moreira E, Li A, Lee R, Rodriguez IR (2001) A family of 12 human genes containing oxysterol-binding domains. Genomics 78:185–196
Raychaudhuri S, Prinz WA (2010) The diverse functions of oxysterol-binding proteins. Annu Rev Cell Dev Biol 26:157–177
Beh CT, Cool L, Phillips J, Rine J (2001) Overlapping functions of the yeast oxysterol-binding protein homologues. Genetics 157:1117–1140
Beh CT, Rine J (2004) A role for yeast oxysterol-binding protein homologs in endocytosis and in the maintenance of intracellular sterol-lipid distribution. J Cell Sci 117:2983–2996
Beh CT, Alfaro G, Duamel G, Sullivan DP, Kersting MC, Dighe S, Kozminski KG, Menon AK (2009) Yeast oxysterol-binding proteins: sterol transporters or regulators of cell polarization? Mol Cell Biochem 326:9–13
Raychaudhuri S, Im YJ, Hurley JH, Prinz WA (2006) Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol 173:107–119
Jansen M, Ohsaki Y, Rita Rega L, Bittman R, Olkkonen VM, Ikonen E (2011) Role of ORPs in sterol transport from plasma membrane to ER and lipid droplets in mammalian cells. Traffic 12:218–231
Georgiev AG, Sullivan DP, Kersting MC, Dittman JS, Beh CT, Menon AK (2011) Osh proteins regulate membrane sterol organization but are not required for sterol movement between the ER and PM. Traffic 12:1341–1355
Santiago-Tirado FH, Bretscher A (2011) Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol 21:515–525
Ortiz D, Medkova M, Walch-Solimena C, Novick P (2002) Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol 157:1005–1015
Santiago-Tirado FH, Legesse-Miller A, Schott D, Bretscher A (2011) PI4P and Rab inputs collaborate in myosin-V-dependent transport of secretory compartments in yeast. Dev Cell 20:47–59
Strahl T, Thorner J (2007) Synthesis and function of membrane phosphoinositides in budding yeast, Saccharomyces cerevisiae. Biochim Biophys Acta 1771:353–404
Walch-Solimena C, Novick P (1999) The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat Cell Biol 1:523–525
Audhya A, Foti M, Emr SD (2000) Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol Biol Cell 11:2673–2689
Bankaitis VA, Phillips S, Yanagisawa L, Li X, Routt S, Xie Z (2005) Phosphatidylinositol transfer protein function in the yeast Saccharomyces cerevisiae. Adv Enzyme Regul 45:155–170
Foti M, Audhya A, Emr SD (2001) Sac1 lipid phosphatase and Stt4 phosphatidylinositol 4-kinase regulate a pool of phosphatidylinositol 4-phosphate that functions in the control of the actin cytoskeleton and vacuole morphology. Mol Biol Cell 12:2396–2411
Manford A, Xia T, Saxena AK, Stefan C, Hu F, Emr SD, Mao Y (2010) Crystal structure of the yeast Sac1: implications for its phosphoinositide phosphatase function. EMBO J 29:1489–1498
Fairn GD, Curwin AJ, Stefan CJ, McMaster CR (2007) The oxysterol binding protein Kes1p regulates Golgi apparatus phosphatidylinositol-4-phosphate function. Proc Natl Acad Sci USA 104:15352–15357
Fang M, Kearns BG, Gedvilaite A, Kagiwada S, Kearns M, Fung MK, Bankaitis VA (1996) Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J 15:6447–6459
Li X, Rivas MP, Fang M, Marchena J, Mehrotra B, Chaudhary A, Feng L, Prestwich GD, Bankaitis VA (2002) Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J Cell Biol 157:63–77
LeBlanc MA, McMaster CR (2010) Lipid binding requirements for oxysterol-binding protein Kes1 inhibition of autophagy and endosome-trans-Golgi trafficking pathways. J Biol Chem 285:33875–33884
Im YJ, Raychaudhuri S, Prinz WA, Hurley JH (2005) Structural mechanism for sterol sensing and transport by OSBP-related proteins. Nature 437:154–158
Drin G, Casella JF, Gautier R, Boehmer T, Schwartz TU, Antonny B (2007) A general amphipathic alpha-helical motif for sensing membrane curvature. Nat Struct Mol Biol 14:138–146
Fairn GD, McMaster CR (2005) Identification and assessment of the role of a nominal phospholipid binding region of ORP1S (oxysterol-binding-protein-related protein 1 short) in the regulation of vesicular transport. Biochem J 387:889–896
Schulz TA, Choi MG, Raychaudhuri S, Mears JA, Ghirlando R, Hinshaw JE, Prinz WA (2009) Lipid-regulated sterol transfer between closely apposed membranes by oxysterol-binding protein homologues. J Cell Biol 187:889–903
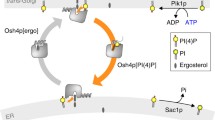
de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G (2011) Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol 195:965–978
Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG (2011) High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J Cell Biol 194:257–275
Behnia R, Munro S (2005) Organelle identity and the signposts for membrane traffic. Nature 438:597–604
Alfaro G, Johansen J, Dighe SA, Duamel G, Kozminski KG, Beh CT (2011) The sterol-binding protein Kes1/Osh4p is a regulator of polarized exocytosis. Traffic 12:1521–1536
Mousley CJ, Yuan P, Gaur NA, Trettin KD, Nile AH, Deminoff SJ, Dewar BJ, Wolpert M, Macdonald JM, Herman PK, Hinnebusch AG, Bankaitis VA (2012) A sterol-binding protein integrates endosomal lipid metabolism with TOR signaling and nitrogen sensing. Cell 148:702–715
Beh CT, McMaster CR, Kozminski KG, Menon AK (2012) A detour for yeast oxysterol binding proteins. J Biol Chem 287:11481–11488
Sebastian TT, Baldridge RD, Xu P, Graham TR (2012) Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta 1821:1068–1077
Surma MA, Klose C, Simons K (2012) Lipid-dependent protein sorting at the trans-Golgi network. Biochim Biophys Acta 1821:1059–1067
Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, Wagner J, Kozak K, Grabner H, Walzer K, Bagnat M, Simons K, Walch-Solimena C (2005) A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc Natl Acad Sci USA 102:17981–17986
Muthusamy BP, Raychaudhuri S, Natarajan P, Abe F, Liu K, Prinz WA, Graham TR (2009) Control of protein and sterol trafficking by antagonistic activities of a type IV P-type ATPase and oxysterol binding protein homologue. Mol Biol Cell 20:2920–2931
Natarajan P, Wang J, Hua Z, Graham TR (2004) Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc Natl Acad Sci USA 101:10614–10619
Natarajan P, Liu K, Patil DV, Sciorra VA, Jackson CL, Graham TR (2009) Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat Cell Biol 11:1421–1426
Graham TR (2004) Flippases and vesicle-mediated protein transport. Trends Cell Biol 14:670–677
Mizuno-Yamasaki E, Medkova M, Coleman J, Novick P (2010) Phosphatidylinositol 4-phosphate controls both membrane recruitment and a regulatory switch of the Rab GEF Sec2p. Dev Cell 18:828–840
Levine T, Loewen C (2006) Inter-organelle membrane contact sites: through a glass, darkly. Curr Opin Cell Biol 18:371–378
Toulmay A, Prinz WA (2011) Lipid transfer and signaling at organelle contact sites: the tip of the iceberg. Curr Opin Cell Biol 23:458–463
Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA (1999) Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol 144:1135–1149
Mogelsvang S, Marsh BJ, Ladinsky MS, Howell KE (2004) Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic 5:338–345
De Matteis MA, Di Campli A, D’Angelo G (2007) Lipid-transfer proteins in membrane trafficking at the Golgi complex. Biochim Biophys Acta 1771:761–768
Hanada K, Kumagai K, Tomishige N, Yamaji T (2009) CERT-mediated trafficking of ceramide. Biochim Biophys Acta 1791:684–691
Levine TP, Munro S (1998) The pleckstrin homology domain of oxysterol-binding protein recognises a determinant specific to Golgi membranes. Curr Biol 8:729–739
Levine TP, Munro S (2002) Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr Biol 12:695–704
Kawano M, Kumagai K, Nishijima M, Hanada K (2006) Efficient trafficking of ceramide from the endoplasmic reticulum to the Golgi apparatus requires a VAMP-associated protein-interacting FFAT motif of CERT. J Biol Chem 281:30279–30288
Loewen CJ, Roy A, Levine TP (2003) A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J 22:2025–2035
Peretti D, Dahan N, Shimoni E, Hirschberg K, Lev S (2008) Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell 19:3871–3884
Perry RJ, Ridgway ND (2006) Oxysterol-binding protein and vesicle-associated membrane protein-associated protein are required for sterol-dependent activation of the ceramide transport protein. Mol Biol Cell 17:2604–2616
Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL (2007) Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA 104:6511–6518
Mohammadi A, Perry RJ, Storey MK, Cook HW, Byers DM, Ridgway ND (2001) Golgi localization and phosphorylation of oxysterol binding protein in Niemann–Pick C and U18666A-treated cells. J Lipid Res 42:1062–1071
Ridgway ND, Dawson PA, Ho YK, Brown MS, Goldstein JL (1992) Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J Cell Biol 116:307–319
Wyles JP, McMaster CR, Ridgway ND (2002) Vesicle-associated membrane protein-associated protein-A (VAP-A) interacts with the oxysterol-binding protein to modify export from the endoplasmic reticulum. J Biol Chem 277:29908–29918
Ngo M, Ridgway ND (2009) Oxysterol binding protein-related Protein 9 (ORP9) is a cholesterol transfer protein that regulates Golgi structure and function. Mol Biol Cell 20:1388–1399
Banerji S, Ngo M, Lane CF, Robinson CA, Minogue S, Ridgway ND (2010) Oxysterol binding protein-dependent activation of sphingomyelin synthesis in the Golgi apparatus requires phosphatidylinositol 4-kinase IIalpha. Mol Biol Cell 21:4141–4150
Lu D, Sun HQ, Wang H, Barylko B, Fukata Y, Fukata M, Albanesi JP, Yin HL (2012) Phosphatidylinositol 4-kinase IIalpha is palmitoylated by Golgi-localized palmitoyltransferases in cholesterol-dependent manner. J Biol Chem 287:21856–21865
Lev S (2010) Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat Rev Mol Cell Biol 11:739–750
Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD (2011) Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell 144:389–401
Goto A, Liu X, Robinson CA, Ridgway ND (2012) Multi-site phosphorylation of oxysterol binding protein (OSBP) regulates sterol binding and activation of sphingomyelin synthesis. Mol Biol Cell 23:3624–3635
Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C, Luini A, Corda D, De Matteis MA (1999) ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol 1:280–287
Nhek S, Ngo M, Yang X, Ng MM, Field SJ, Asara JM, Ridgway ND, Toker A (2010) Regulation of oxysterol-binding protein Golgi localization through protein kinase D-mediated phosphorylation. Mol Biol Cell 21:2327–2337
Kumagai K, Kawano M, Shinkai-Ouchi F, Nishijima M, Hanada K (2007) Interorganelle trafficking of ceramide is regulated by phosphorylation-dependent cooperativity between the PH and START domains of CERT. J Biol Chem 282:17758–17766
Lange Y, Ye J, Steck TL (2004) How cholesterol homeostasis is regulated by plasma membrane cholesterol in excess of phospholipids. Proc Natl Acad Sci USA 101:11664–11667
Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Gruenberg J (2002) Separation and characterization of late endosomal membrane domains. J Biol Chem 277:32157–32164
Rosenbaum AI, Maxfield FR (2011) Niemann–Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem 116:789–795
Acknowledgments
We thank Christine Doucet for critical comments on the manuscript. Our work is financed by the ERC (Advanced Grant 268888), the CNRS and the ANR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mesmin, B., Antonny, B. & Drin, G. Insights into the mechanisms of sterol transport between organelles. Cell. Mol. Life Sci. 70, 3405–3421 (2013). https://doi.org/10.1007/s00018-012-1247-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1247-3