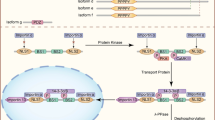
Abstract
Syntrophins are a family of cytoplasmic membrane-associated adaptor proteins, characterized by the presence of a unique domain organization comprised of a C-terminal syntrophin unique (SU) domain and an N-terminal pleckstrin homology (PH) domain that is split by insertion of a PDZ domain. Syntrophins have been recognized as an important component of many signaling events, and they seem to function more like the cell’s own personal ‘Santa Claus’ that serves to ‘gift’ various signaling complexes with precise proteins that they ‘wish for’, and at the same time care enough for the spatial, temporal control of these signaling events, maintaining overall smooth functioning and general happiness of the cell. Syntrophins not only associate various ion channels and signaling proteins to the dystrophin-associated protein complex (DAPC), via a direct interaction with dystrophin protein but also serve as a link between the extracellular matrix and the intracellular downstream targets and cell cytoskeleton by interacting with F-actin. They play an important role in regulating the postsynaptic signal transduction, sarcolemmal localization of nNOS, EphA4 signaling at the neuromuscular junction, and G-protein mediated signaling. In our previous work, we reported a differential expression pattern of alpha-1-syntrophin (SNTA1) protein in esophageal and breast carcinomas. Implicated in several other pathologies, like cardiac dys-functioning, muscular dystrophies, diabetes, etc., these proteins provide a lot of scope for further studies. The present review focuses on the role of syntrophins in membrane targeting and regulation of cellular proteins, while highlighting their relevance in possible development and/or progression of pathologies including cancer which we have recently demonstrated.







Similar content being viewed by others
References
Lee SH, Sheng M (2000) Development of neuron–neuron synapses. Curr Opin Neurobiol 19(1):125–131
Sheng M, Sala C (2001) PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci 24:1–29
Adams ME, Dwyer TM, Dowler LL, White RA, Froehner SC (1995) Mouse alpha 1- and beta 2-syntrophin gene structure, chromosome localization, and homology with a discs large domain. J Biol Chem 270(43):25859–25865
Ahn AH, Yoshida M, Anderson MS, Freener CA, Selig S, Hagiwara Y, Ozawa E, Kunkel LM (1994) Cloning of human basic A1, a distinct 59-kDa dystrophin-associated protein encoded on chromosome 8q23–24. Proc Natl Acad Sci USA 91:4446–4450
Froehner SC, Adams ME, Peters MF, Gee SH (1997) Syntrophins: modular adapter proteins at the neuromuscular junction and the sarcolemma. Soc Gen Physiol Ser 52:197–207
Froehner SC (1984) Peripheral proteins of postsynaptic membranes from torpedo electric organ identified with monoclonal antibodies. J Cell Biol 99:88–96
Ahn AH, Freener CA, Gussoni E, Yoshida M, Ozawa E, Kunkel LM (1996) The three human syntrophin genes are expressed in diverse tissues, have distinct chromosomal location, and each bind to dystrophin and its relatives. J Biol Chem 271:2724–2730
Froehner SC, Murnane AA, Tobler M, Peng HB, Sealock R (1987) A postsynaptic Mr 58,000 (58 K) protein concentrated at acetylcholine receptor-rich sites in Torpedo electroplaques and skeletal muscle. J Cell Biol 104:1633–1646
Kramarcy NR, Vidal A, Froehner SC, Sealock R (1994) Association of utrophin and multiple dystrophin short forms with the mammalian M(r) 58,000 dystrophin-associated protein (syntrophin). J Biol Chem 269:2870–2876
Hoffman EP, Brown RH, Kunkel LM (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51:919–928
Campbell KP, Kahl SD (1989) Association of dystrophin and an integral membrane glycoprotein. Nature 338:259–262
Adams ME, Butler MH, Dwyer TM, Peters MF, Murnane AA, Froehner SC (1993) Two forms of mouse syntrophin, a 58 kd dystrophin-associated protein, differ in primary structure and tissue distribution. Neuron 11:531–540
Yang B, Ibraghimov-Beskrovnaya O, Moomaw CR, Slaughter CA, Campbell KP (1994) Heterogeneity of the 59-kDa dystrophin associated protein revealed by cDNA cloning and expression. J Biol Chem 269:6040–6044
Butler MH, Douville K, Murnane AA, Kramarcy NR, Cohen JB, Sealock R, Froehner SC (1992) Association of the Mr 58,000 postsynaptic protein of electric tissue with Torpedo dystrophin and the Mr 87,000 postsynaptic protein. J Biol Chem 267:6213–6218
Ahn AH, Kunkel LM (1993) The structural and functional diversity of dystrophin. Nat Genet 3:283–291
Piluso G, Mirabella M, Ricci E, Belsito A, Abbondanza C, Servidei S, Puca AA, Tonali P, Puca GA, Nigro V (2000) Gamma-1 and gamma-2-syntrophins, two novel dystrophin-binding proteins localized in neuronal cells. J Biol Chem 275:15851–15860
Ahn AH, Kunkel LM (1995) Syntrophin binds to an alternatively spliced exon of dystrophin. J Cell Biol 128:363–371
Kramarcy NR, Sealock R (2000) Syntrophin isoforms at the neuromuscular junction: developmental time course and differential localization. Mol Cell Neurosci 15:262–274
Peters MF, Adams ME, Froehner SC (1997) Differential association of syntrophin pairs with the dystrophin complex. J Cell Biol 138(1):81–93
Bhat HF, Baba RA, Bashir M, Saeed S, Kirmani D, Wani MM, Wani NA, Wani KA, Khanday FA (2010) Alpha-1-syntrophin protein is differentialy expressed in human cancers. Biomarkers 16:31–36
Alessi A, Bragg AD, Percival JM, Yoo J, Albrecht DE, Froehner SC, Adams ME (2006) γ-Syntrophin scaffolding is spatially and functionally distinct from that of the α/β syntrophins. Exp Cell Res 312:3084–3095
Peters MF, Kramarcy NR, Sealock R, Froehner SC (1994) β-2 syntrophin: localization at the neuromuscular junction in skeletal muscle. Neuro Report 5:1577–1580
Sealock R, Butler MH, Kramarcy NR, Gao K, Murnane AA, Douville K, Froehner SC (1991) Localization of dystrophin relative to acetylcholine receptor domains in electric tissue and adult and cultured skeletal muscle. J Cell Biol 113:1133–1144
Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC (2000) Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol 150:1385–1398
Way M, Pope B, Cross RA, Kendrick-Jones J, Weeds AG (1992) Expression of the N-terminal domain of dystrophin in E.coli and demonstration of binding to F-actin. FEBS Lett 301:243–245
Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS (1996) Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84:757–767
Okumura H, Okumura N, Iwamatsu A, Buijs RM, Romijn HJ et al (1999) Interaction of neuronal nitric oxide synthase with alpha-1-syntrophin in rat brain. J Biol Chem 247:11736–11741
Lumeng C, Phelps S, Crawford GE, Walden PD, Barald K, Chamberlain JS (1999) Interactions between beta 2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat Neurosci 2:611–617
Hogan A, Shepherd L, Chabot J, Quenneville S, Prescott SM, Topham MK, Gee SH (2001) Interaction of gamma 1-syntrophin with diacylglycerol kinase-zeta. Regulation of nuclear localization by PDZ interactions. J Biol Chem 276(28):26526–26533
Saraste M, Sibbald PR, Wittinghofer A (1990) The P-loop-a common motif in ATP- and GTP-binding proteins. Trends Biochem Sci 15:430–434
Albrecht DE, Froehner SC (2002) Syntrophins and dystrobrevins: defining the dystrophin scaffold at synapses. Neurosignals 11:123–129
Lemmon MA, Ferguson KM (2000) Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J 350(Part 1):1–18
Musacchio A, Gibson T, Rice P, Thompson J, Saraste M (1993) The PH-domain: a common piece in the structural patchwork of signaling proteins. Trends Biochem Sci 18:343–348
Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE (2000) Myosin-X, a novel myosin with pleckstrin homology domains, associated with regions of dynamic actin. J Cell Sci 113:3439–3451
Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95:5857–5864
Gibson TJ, Hyvonen M, Musacchio A, Saraste M, Birney E (1994) PH domain: the first anniversary. Trends Biochem Sci 19:349–353
Chang JS, Kim SK, Kwon TK, Bae SS, Min DS, Lee YH, Kim SO, Seo JK, Choi JH, Suh PG (2005) Pleckstrin homology domains of phospholipase C-{gamma}1 directly interact with {beta}-tubulin for activation of phospholipase C-{gamma}1 and reciprocal modulation of {beta}-tubulin function in microtubule assembly. J Biol Chem 280:6897–6905
Chang JS, Seok H, Kwon TK, Min DS, Ahn BH, Lee YH, Suh JW, Kim JW, Iwashita S, Omori A, Ichinose S, Numata O, Seo JK, Oh YS, Suh PG (2002) Interaction of elongation factor-1alpha and pleckstrin homology domain of phospholipase C-gamma 1 with activating its activity. J Biol Chem 277:19697–19702
Jing Y, Wenyu W, Weiguang X, Jia-fu L, Marvin EA, Froehner SC, Mingjie Z (2005) Structure of the split PH domain and distinct lipid binding properties of the PH–PDZ supramodule of α-syntrophin. EMBO J 24:3985–3995
Iwata Y, Pan Y, Yoshida T, Hanada H, Shigekawa M (1998) Bidirectional signaling between sarcoglycans and the integrin adhesion system in cultured L6 Myocytes. FEBS Lett 423:173–177
Newbell BJ, Anderson JT, Jarrett HW (1997) Ca2+-calmodulin binding to mouse α-1 syntrophin: syntrophin is also a Ca2+-binding protein. Biochemistry 36:1295–1305
Oak SA, Jarrett HW (2000) The oligomerization of mouse α-1-syntrophin and self-association of its pleckstrin homology domain 1. Biochemistry 39:8870–8877
Chockalingam PS, Gee SH, Jarrett HW (1999) Pleckstrin homology domain 1 of mouse α-1-syntrophin binds phosphatidylinositol 4,5-bisphosphate. Biochemistry 38:5596–5602
Van-Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH (2005) Phospholipase C gamma1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature 434:99–104
Gardiol D (2012) PDZ-containing proteins as targets in human pathologies. FEBS J 279:3529
Feng W, Zhang M (2009) Organization and dynamicsof PDZ-domain-related supramodules in the postsynaptic density. Nat Rev Neurosci 10:87–99
Nourry C, Grant SG, Borg JP (2003) PDZ domain proteins: plug and play. Sci STKE2003 RE7
Chimura T, Launey T, Ito M (2011) Evolutionarily conserved bias of amino acid usage refines the definition of PDZ-binding motif. BMC Genomics 12:300
Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA (1999) Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science 284:812–815
Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R (1996) Crystal structures of a complexed and peptide-free membrane proteinbinding domain: molecular basis of peptide recognition by PDZ. Cell 85:1067–1076
Schultz J, Hoffmuuller U, Krause G, Ashurst J, Macias MJ, Schmieder P, Schneider-Mergener J, Oschkinat H (1998) Specific interactions between the syntrophin PDZ domain and voltage-gated sodium channels. Nature Struct Biol 5:19–24
Wiedemann U, Boisguerin P, Leben R, Leitner D, Kraus G, Moelling K, Volkmer-Engert R, Oschkinat H (2004) Quantification of PDZ domain specificity, prediction of ligand affinity and rational design of super binding peptides. J Mol Biol 343:703–718
Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M (1995) Clustering of Shaker-type K1 channels by direct interaction with the PSD-95/SAP90 family of membrane associated guanylate kinases. Nature 378:85–88
Kornau HC, Schenker LT, Kennedy MB, Seeburg PH (1995) Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science 269:1737–1740
Adams ME, Mueller HA, Froehner SC (2001) In vivo requirement of the {alpha}-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol 155:113–122
Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME (2001) Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA 98:14108–14113
Gee SH, Madhavan R, Levinson SR, Caldwell JH, Sealock R, Froehner SC (1998) Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J Neurosci 18:128–137
Ou Y, Strege P, Miller SM, Makielski J, Ackerman M, Gibbons SJ, Farrugia G (2003) Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J Biol Chem 278:1915–1923
Connors NC, Adams ME, Froehner SC, Kofuji P (2004) The potassium channel Kir4.1 associates with the dystrophin–glycoprotein complex via alpha-syntrophin in glia. J Biol Chem 279:28387–28392
Leonoudakis D, Conti LR, Anderson S, Radeke CM, McGuire LM, Adams ME, Froehner SC, Yates JR, Vandenberg CA (2004) Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)- associated proteins. J Biol Chem 279:22331–22346
Vandebrouck A, Sabourin J, Rivet J, Balghi H, Sebille S, Kitzis A, Raymond G, Cognard C, Bourmeyster N, Constantin B (2007) Regulation of capacitative calcium entries by α-1-syntrophin: association of TRPC1 with dystrophin complex and the PDZ domain of α-1-syntrophin. FASEB J. 21:608–617
Hasegawa M, Cuenda A, Spillantini MG, Thomas GM, Buee-Scherrer V, Cohen P, Goedert M (1999) Stress-activated protein kinase-3 interacts with the PDZ domain of alpha1-syntrophin. A mechanism for specific substrate recognition. J Biol Chem 274:12626–12631
Buechler C, Boettcher A, Bared SM, Probst MC, Schmitz G (2002) The carboxy terminus of the ATP-binding cassette transporter A1 interacts with a beta-2-syntrophin/utrophin complex. Biochem Biophys Res Commun 293:759–765
Torchio GM, Ermacora MR, Sica MP (2012) Equilibrium unfolding of the PDZ domain of β-2-syntrophin. Biophys J 102(12):2835–2844
Schubert S, Knoch KP, Ouwendijk J, Mohammed S, Bodrov Y, Jager M, Altkruger A, Wegbrod C, Adams ME, Kim Y, Froehner SC, Jensen ON, Kalaidzidis Y, Solimena M (2010) β-2-Syntrophin is a Cdk5 substrate that restrains the motility of insulin secretory granules. PLoS ONE 5(9):e12929
Trejo JA (2005) Internal PDZ ligands: novel endocytic recycling motifs for G protein-coupled receptors. Mol Pharmacol 67:1388–1390
Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, Huitfeldt HS, Dahl SG, Attramadal H (2005) Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol 67:1581–1590
Gage RM, Matveeva EA, Whiteheart SW, Von-Zastrow M (2005) Type I PDZ ligands are sufficient to promote rapid recycling of G-protein coupled receptors independent of binding to NSF. J Biol Chem 280:3305–3313
Heydorn A, Sondergaard BP, Ersboll B, Holst B, Nielson FC, Haft CR, Whistler J, Schwartz TW (2004) A library of 7TM receptor C-terminal tails. J Biol Chem 279:54291–54303
Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB (1994) Crystal structure at 2.2 A resolution of the pleckstrin homology domain from human dynamin. Cell 79:199–209
Fushman D, Cahill S, Lemmon MA, Schlessinger J, Cowburn D (1995) Solution structure of pleckstrin homology domain of dynamin by heteronuclear NMR spectroscopy. Proc Natl Acad Sci USA 92:816–820
Macias MJ, Musacchio A, Ponstingl H, Nilges M, Saraste M, Oschkinat H (1994) Structure of the pleckstrin homology domain from beta-spectrin. Nature 369:675–677
Yoon HS, Hajduk PJ, Petros AM, Olejniczak ET, Meadows RP, Fesik SW (1994) Solution structure of a pleckstrin-homology domain. Nature 369:672–675
Zhao C, Yu DH, Shen R, Feng GS (1999) Gab2, a new pleckstrin homology domain-containing adapter protein, acts to uncouple signaling from ERK kinase to Elk-1. J Biol Chem 274:19649–19654
Kachinsky AM, Froehner SC, Milgram SL (1999) A PDZ-containing scaffold related to the dystrophin complex at the basolateral membrane of epithelial cells. J Cell Biol 145:391–402
Suzuki A, Yoshida M, Ozawa E (1995) Mammalian alpha 1- and beta 1-syntrophin bind to the alternative splice-prone region of the dystrophin COOH terminus. J Cell Biol 128:373–381
Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contractions. Proc Natl Acad Sci 90:3710–3714
Ambramovici H, Hogan AB, Obagi C, Topham MK, Gee SH (2003) Diacylglycerol kinase-ζ localization in skeletal muscles is regulated by phosphorylation and interaction with syntrophins. Mol Biol Cell 14:4499–4511
Hogan A, Yakubchyk Y, Josee-Chabot J, Obagi C, Daher E, Maekawa K, Gee SH (2004) The phosphoinositol 3,4-bisphosphate-binding protein TAPP1 interacts with syntrophins and regulates actin cytoskeletal organization. J Biol Chem 51:53717–53724
Cantley LC (2002) The phosphoinositide 3-kinase Pathway. Science 296:1655–1657
Iwata Y, Sampaolesi M, Shigekawa M, Wakabayashi S (2004) Syntrophin is an actin-binding protein the cellular localization of which is regulated through cytoskeletal reorganization in skeletal muscle cells. Eur J Cell Biol 83:555–565
Topham MK, Bunting M, Zimmerman GA, McIntyre TM, Blackshear PJ, Prescott SM (1998) Protein kinase C regulates the nuclear localization of diacylglycerol kinase-ζ. Nature 394:697–700
Kimber WA, Trinkle-Mulcahy L, Cheung PC, Deak M, Marsden LJ, Kieloch A, Watt S, Javier RT, Gray A, Downes CP, Lucocq JM, Alessi DR (2002) Evidence that the tandem-pleckstrin-homology-domain-containing protein TAPP1 interacts with Ptd(3,4)P2 and the multi-PDZ-domain-containing protein MUPP1 in vivo. Biochem J 361:525–536
Dowler S, Currie RA, Downes CP, Alessi DR (1999) DAPP1: a dual adaptor for phosphotyrosine and 3-phosphoinositides. Biochem J 342:7–12
Dowler S, Currie RA, Campbell DG, Deak M, Kular G, Downes CP, Alessi DR (2000) Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem J 351:19–31
Marshall AJ, Krahn AK, Ma K, Duronio V, Hou S (2002) TAPP1 and TAPP2 Are Targets of Phosphatidylinositol 3-Kinase Signaling in B Cells: sustained Plasma Membrane Recruitment Triggered by the B-Cell Antigen Receptor. Mol Cell Biol 22:5479–5491
Marshall AJ, Niiro H, Lerner CG, Yun TJ, Thomas S, Disteche CM, Clark EA (2000) Dual adapter for phosphotyrosine and 3-phosphotyrosine and 3-phosphoinositide (hDAPP1) (B cell adapter molecule of 32 kDa) (Blymphocyte adapter protein Bam32). J Exp Med 191:1319–1332
Newey SE, Benson MA, Ponting CP, Davies KE, Blake DJ (2000) Alternative splicing of dystrobrevin regulates the stoichiometry of syntrophin binding to the dystrophin protein complex. Curr Biol 10:1295–1298
Buccione R, Orth JD, McNiven MA (2004) Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 5:647–657
Bruno G, Jean-Sebastien R, Andrea AD, Romina B, Christophe B, Patrick R, Hans-Anton L, Thierry P, Hugues A (2006) Cardiac sodium channel Nav1.5 is regulated by a multiprotein complex composed of syntrophins and dystrophin. Circ Res 99:407–414
Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS (1995) Localization of MIWC and GLIP water channel homologs in neuro-muscular, epithelial and glandular tissues. J Cell Sci 108:2993–3002
Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP (1997) Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17:171–180
Agre P (2006) The aquaporin water channels. Proc Am Thorac Soc 3(1):5–13
Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S (1995) Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol 269:F775–F785
Blake DJ, Hawkes R, Benson MA, Beesley PW (1999) Different dystrophin-like complexes are expressed in neurons and glia. J Cell Biol 147:645–658
Durbeej M, Campbell KP (1999) Biochemical characterization of the epithelial dystroglycan complex. J Biol Chem 274:26609–26616
Tian M, Jacobson C, Gee SH, Campbell KP, Carbonetto S, Jucker M (1996) Dystroglycan in the cerebellum is a laminin alpha 2-chain binding protein at the glial-vascular interface and is expressed in Purkinje cells. Eur J Neurosci 8:2739–2747
Fanning AS, Anderson JM (1999) PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest 103:767–772
Neely JD, Christensen BM, Nielsen S, Agre P (1999) Heterotetrameric composition of aquaporin-4 water channels. Biochemistry 38(34):11156–11163
Poopalasundaram S, Knott C, Shamotienko OG, Foran PG, Dolly O, Ghiani CA, Gallo V, Wilkin GP (2000) Glial heterogeneity in expression of the inwardly rectifying K(+) channel, Kir4.1, in adult rat CNS. Glia 30:362–372
Ishii M, Horio Y, Tada Y, Hibino H, Inanobe A, Ito M, Yamada M, Gotow T, Uchiyama Y, Kurachi Y (1997) Expression and clustered distribution of an inwardly rectifying potassium channel, KAB2/Kir4.1, on mammalian retinal Muller cell membrane: their regulation by insulin and laminin signals. J Neurosci 17:7725–7735
Amedee T, Robert A, Coles JA (1997) Potassium homeostasis and glial energy metabolism. Glia 21:46–55
Kofuji P, Connors NC (2003) Molecular substrates of potassium spatial buffering in glial cells. Mol Neurobiol 28:195–208
Sabourin J, Cognard C, Constantin B (2009) Regulation by scaffolding proteins of canonical transient receptor potential channels in striated muscle. J Muscle Res Cell Motil 30:289–297
Vandebrouck C, Martin D, Colson-Van SM, Debaix H, Gailly P (2002) Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibres. J Biol Chem 158:1089–1096
Stiber JA, Zhang ZS, Burch J, Eu JP, Zhang S, Truskey GA, Seth M, Yamaguchi N, Meissner G, Shah R, Worley PF, Williams RS, Rosenberg PB (2008) Mice lacking Homer-1 exhibit a skeletal myopathy characterized by abnormal transient receptor potential channel activity. Mol Cell Biol 28:2637–2647
Madhavan R, Massom LR, Jarrett HW (1992) Calmodulin specifically binds three proteins of the dystrophin-glycoprotein complex. Biochem Biophys Res Commun 185:753–759
Cohen P (1997) The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol 7:353–361
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642
Iglesias T, Cabrera-Poch N, Mitchell MP, Naven TJ, Rozengurt E, Schiavo G (2000) Identification and cloning of Kidins220, a novel neuronal substrate of protein kinase D. J Biol Chem 275:40048–40056
Kong HY, Boulter J, Weber JL, Lai C, Chao MV (2001) An evolutionarily conserved transmembrane protein that is a novel downstream target of neurotrophin and ephrin receptors. J Neurosci 21:176–185
Shuo L, Yu C, Kwok OL, Juan CA, Froehner SC, Marvin E, Adams ME, Moses V, Chao NY (2005) α-1-syntrophin regulates ARMS localization at the neuromuscular junction and enhances EphA 4 signaling in an ARMS-dependent manner. J Cell Biol 169(5):813–824
Arevalo JC, Yano H, Teng KK, Chao MV (2004) A unique pathway for sustained neurotrophin signaling through an ankyrin-rich membrane-spanning protein. EMBO J 23:2358–2368
Oak SA, Russo K, Petrucci TC, Jarrett HW (2001) Mouse alpha1-syntrophin binding to Grb2: further evidence of a role for syntrophin in cell signaling. Biochemistry 40:11270–11278
Zhou YW, Oak SA, Senogles SE, Jarrett HW (2005) Laminin-R1 globular domains three and four induce heterotrimeric G-protein binding to R-syntrophin’s PDZ domain. Am J Physiol Cell Physiol 288:C377–C388
Madhavan R, Jarrett HW (1995) Interactions between dystrophin glycoprotein complex proteins. Biochemistry 34:12204–12209
Ervasti JM, Campbell KP (1993) A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 122:809–823
Jarrett HW, Foster JL (1995) Alternate binding of actin and calmodulin to multiple sites on dystrophin. J Biol Chem 270:5578–5586
Vidal M, Goudreau N, Cornille F, Cussac D, Gincel E, Garbay C (1999) Molecular and cellular analysis of Grb2 SH3 domain mutants: interaction with Sos and dynamin. J Mol Biol 290:717–730
Oak SA, Zhou YW, Jarrett HW (2003) Skeletal muscle signaling pathway through the dystrophin glycoprotein complex and Rac1. J Biol Chem 278:39287–39295
Yang B, Jung D, Rafael JA, Chamberlain JS, Campbell KP (1995) Identification of alpha-syntrophin binding to syntrophin triplet, dystrophin, and utrophin. J Biol Chem 270(10):4975–4978
Khanday FA, Yamamori T, Singh IM, Zhang Z, Bugayenko A, Naqvi A, Santhanum L, Nabi N, Kasuno K, Day BW, Irani K (2006) Rac1 Leads to Phosphorylation-dependent increase in stability of the p66shc adaptor protein: role in Rac1-induced oxidative stress. Mol Biol Cell 17:122–129
Khanday FA, Santhanam L, Kasuno K, Yamamori T, Naqvi A, Dericio J, Bugazenko A, Mattagajasingh I, Disanza A, Scita G, Irani K (2006) SOS-mediated activation of Rac1 by p66shc. J Cell Biol 172:817–822
Reece J, Campbell N (2002) Biology. Benjamin Cummings, San Francisco
Akiko O, Katsuya N, Nobuaki O (2008) Interaction of α-1-syntrophin with multiple isoforms of heterotrimeric G protein a subunits. FEBS J 275:22–33
Yatani A, Codina J, Imoto Y, Reeves JP, Birnbaumer L, Brown AM (1987) A G-protein directly regulates mammalian cardiac calcium channels. Science 238:1288–1292
Stevens FC (1983) Calmodulin: an introduction. Can J Biochem Cell Biol 61(8):906–910
Chin D, Means AR (2000) Calmodulin: a prototypical calcium sensor. Trends Cell Biol 10(8):322–328
Nathan C, Xie QW (1994) Nitric oxide synthases: roles, tolls, and controls. Cell 78:915–918
Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM (2002) Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 416(6878):337–339
Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B (2003) Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res 92:e52–e59
Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C (2004) Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet 363:1365–1367
Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS (1995) Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82:743–752
Judith CW, Angel LA, Tamer MA, Cassandra LH, Fiona HS, Aly OZ, Delvac O, Elizabeth JC, Mamta HB, Michael E, Ludwig N (2006) The sarcolemmal calcium pump, α-1-syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J Biol Chem 281:23341–23348
Xu XZ, Choudhury A, Li X, Montell C (1998) Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins. J Cell Biol 142:545–555
Schuh K, Uldrijan S, Telkamp M, Rothlein N, Neyses L (2001) The plasma membrane calmodulin-dependent calcium pump: a major regulator of nitric oxide synthase. J Cell Biol 155:201–205
Wang Y, Oram JF (2002) Unsaturated fatty acids inhibit cholesterol efflux from macrophages by increasing degradation of atp-binding cassette transporter a1. J Biol Chem 277:5692–5697
Attie AD, Kastelein JP, Hayden MR (2001) Pivotal role of ABCA1 in reverse cholesterol transport influencing HDL levels and susceptibility to atherosclerosis. J Lipid Res 42:1717–1726
Francis GA, Knopp RH, Oram JF (1995) Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J Clin Investig 96:78–87
Remaley AT, Schumacher UK, Stonik JA, Farsi BD, Nazih H, Brewer HB (1997) Decreased reverse cholesterol transport from Tangier disease fibroblasts: acceptor specificity and effect of Brefeldin on lipid efflux. Arterioscler Thromb Vasc Biol 17:1813–1821
Takahashi Y, Smith JD (1999) Cholesterol efflux to apolipoprotein AI involves endocytosis and resecretion in a calcium-dependent pathway. Proc Natl Acad Sci USA 96:11358–11363
Youichi M, Tomohiro O, Shinobu K, Akiko F, Kenya S, Michihiro I, Toshifumi Y, Shin’ichi T, Teruo A, Michinori M, Noriyuki K, Kazumitsu U (2004) α-1-Syntrophin modulates turnover of ABCA1. J Biol Chem 279:15091–15095
Rigot V, Hamon Y, Chambenoit O, Aliber M, Duverger N, Chimini G (2002) Distinct sites on ABCA1 control distinct steps required for cellular release of phospholipids. J Lipid Res 43:2077–2086
Wang N, Chen W, Linsel-Nitschke P, Martinez LO, Agerholm-Larsen B, Silver DL, Tall AR (2003) A PEST sequence in ABCA1 regulates degradation by calpain protease and stabilization of ABCA1 by apoA-I. J Clin Investig 111:99–107
Okuhira K, Fitzgerald ML, Sarracino DA, Manning JJ, Bell SA, Goss JL, Freeman MW (2005) Purification of ATP-binding cassette transporter A1 and associated binding proteins reveals the importance of beta1-syntrophin in cholesterol efflux. J Biol Chem 280(47):39653–39664
Straub V, Campbell KP (1997) Muscular dystrophies and the dystrophin glycoprotein complex. Curr Opin Neurol 10:168–175
Matsumura K, Tome FM, Collin H, Azibi K, Chaouch M, Kaplan JC, Fardeau M, Campbell KP (1992) Deficiency of the 50K dystrophin associated glycoprotein in severe childhood autosomal recessive muscular dystrophy. Nature 359:320–322
Adams ME, Kramarcy N, Fukuda T, Engel AG, Sealock R, Froehner SC (2004) Structural abnormalities at neuromuscular synapses lacking multiple syntrophin isoforms. J Neurosci 24:10302–10309
Compton AG, Cooper ST, Hill PM, Yang N, Froehner SC, North KN (2005) The syntrophin-dystrobrevin sub-complex in human neuromuscular disorders. J Neuropathol Exp Neurol 64(4):350–361
Kim MJ, Hwang SH, Lim JA, Froehner SC, Adams ME, Kim HS (2010) α-Syntrophin modulates myogenin expression in differentiating myoblasts. PLoS ONE 5(12):e15355
Wakayama Y, Inoue, Kojima M, Jimi T, Yamashita S, Kumagai T, Shibuya S, Hara H, Oniki H (2006) Altered alpha1-syntrophin expression in myofibers with Duchenne and Fukuyama muscular dystrophies. Histol Histopathol 21(1):23–34
Nakamori M, Kimura T, Kubota T, Matsumura T, Sumi H, Fujimura H, Takahashi MP, Sakoda S (2008) Aberrantly spliced alpha-dystrobrevin alters alpha-syntrophin binding in myotonic dystrophy type 1. Neurology 70(9):677–685
Alderton JM, Steinhardt RA (2000) Calcium influx through leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 275:9452–9460
Wu G, Ai T, Kim JJ, Mohapatra B, Xi Y, Li Z, Abbasi S, Purevjav E, Samani K, Ackerman MJ, Qi M, Moss AJ, Shimizu W, Towbin JA, Cheng J, Vatta M (2008) Alpha-1-syntrophin mutation and the long-QT syndrome: a disease of sodium channel disruption. Circ Arrhythm Electrophysiol 1(3):193–201
Cheng J, Van Norstrand DW, Medeiros-Domingo A, Valdivia C, Tan B, Ye B, Kroboth S, Vatta M, Tester DJ, January CT, Makielski JC, Ackerman MJ (2009) α1-Syntrophin mutations identified in sudden infant death syndrome cause an increase in late cardiac sodium current. Circ Arrhythm Electrophysiol 2:667–676
Lehnart SE, Ackerman MJ, Benson DW, Brugada R, Clancy CE, Donahue JK, George AL, Grant AO, Groft SC, January CT, Lathrop DA. Lederer WJ, Makielski JC, Mohler PJ, Moss A, Jeanne M, Olson TM, Przywara DA, Towbin JA, Wang LH, Marks AR (2007) Inherited arrhythmias: A National Heart, Lung, and Blood Institute and Office of Rare Diseases workshop consensus report about the diagnosis, phenotyping, molecular mechanisms, and therapeutic approaches for primary cardiomyopathies of gene mutations affecting ion channel function. Circulation 116: 2325–2345
Oceandy D, Cartwright EJ, Emerson M, Prehar S, Baudoin FM, Zi M, Alatwi N, Venetucci L, Schuh K, Judith CW, Armesilla AL, Neyses L (2007) Neuronal nitric oxide synthase signaling in the heart is regulated by the sarcolemmal calcium pump 4b. Circulation 115:483–492
Wang Y, Kodani E, Wang J, Zhang SX, Takano H, Tang XL, Bolli R (2004) Cardioprotection during the final stage of the late phase of ischemic preconditioning is mediated by neuronal NO synthase in concert with cyclooxygenase-2. Circ Res 95:84–91
Ueda K, Valdivia C, Medeiros-Domingo A, David J, Tester DJ (2008) Syntrophin mutation associated with long QT syndrome through activation of the nNOS–CN5A macromolecular complex. Proc Natl Acad Sci USA 105(27):9355–9360
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of diabetes epidemic. Nature 414:782–787
Diamond J (2003) The double puzzle of diabetes. Nature 423:599–602
Trajkovski M, Mziaut H, Schubert S, Kalaidzidis Y, Altkruger A, Solimena M (2008) Regulation of insulin granule turnover in pancreatic β-cells by cleaved ICA512. J Biol Chem 283:33719–33729
Ort T, Maksimova E, Dirkx R, Kachinsky AM, Berghs S, Froehner SC, Solimena M (2000) The receptor tyrosine phosphatase-like protein ICA512 binds the PDZ domains of β2-syntrophin and nNOS in pancreatic β-cells. Eur J Cell Biol 79:621–630
Ort T, Voronov S, Guo J, Zawalich K, Freohner SC, Zawalich W, Solimena M (2001) Dephosphorylation of β-2-syntrophin and Ca2+/μ-calpain-mediated cleavage of ICA512 upon stimulation of insulin secretion. EMBO J 20:4013–4023
Zhong H, Minneman KP (1999) Alpha1-adrenoceptor subtypes. Eur J Pharmacol 375:261–276
Hague C, Chen Z, Uberti M, Minneman KP (2003) Alpha1-adrenergic receptor subtypes: non-identical triplets with different dancing partners? Life Sci 74(4):411–418
Zhongjian C, Chris H, Randy AH, Kenneth PM (2006) Syntrophins regulate α_1D-adrenergic receptors through a PDZ domain-mediated interaction. J Biol Chem 281:12414–12420
McCloskey DT, Rokosh DG, O’Connell TD, Keung EC, Simpson PC, Baker AJ (2002) α1-adrenoceptor subtypes mediate negative inotropy in myocardium from α1A/C-knockout and wild type mice. J Mol Cell Cardiol 34:1007–1017
Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G (2002) The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Chromatogr A 109:765–775
Tanoue A, Koba M, Miyawaki S, Koshimizu TA, Hosoda C, Oshikawa S, Tsujimoto G (2002) Role of the α1D-adrenergic receptor in the development of salt-induced hypertension. Hypertension 40:101–106
Yamakawa H, Oyama S, Mitsuhashi H, Sasagawa N, Uchino S, Kohsaka S, Ishiura S (2007) Neuroligins 3 and 4X interact with syntrophin-γ2 and the interactions are affected by autism-related mutations. Biochem Biophy Res Commun 355:41–46
Ichtchenko K, Halta Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC (1995) Neuroligin 1: a splice site-specific ligand for beta-neuroxins. Cell 81:435–443
Graf ER, Zhang X, Jin SX, Linhoff MW, Criag AM (2004) Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via Neuroligins. Cell 119:1013–1026
Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P (2003) Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci 6:708–716
Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gilberg IC, Soderstrom H, Giros B, Leboyer M, Gilberg C et al (2003) Mutations of the X-linked genes encoding Neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 34:27–29
Facciuto F, Cavatorta AL, Valdano MB, Marziali F, Gardiol D (2012) Differential expression of PDZ domain containing proteins in human diseases-challenging topics and novel issues. FEBS J 279:3538–3548
Pim D, Bergant M, Boon SS, Ganti K, Kranjec C, Massimi P, Subbaiah VK, Thomas M, Tomaic V, Banks L (2012) Human papillomaviruses and the specificity of PDZ domain targeting. FEBS J 279:3530–3537
Wiseman SM, Griffith O, Leung S, Masoudi H, Phang PT, Jones SJ, Hakama M (2008) Syntrophin expression predicts colon cancer outcome. Am Assoc Cancer Res
Carinci F, Lo Muzio L, Piattelli A, Rubini C, Chiesa F, Ionna F, Palmieri A, Maiorano E, Pastore A, Laino G, Dolci M, Pezzetti F (2005) Potential markers of tongue tumor progression selected by cDNA microarray. Int J Immunopathol Pharmacol 18(3):513–524
Acknowledgment
This work was supported by the Department of Biotechnology, Ministry of Science and Technology, Government of India, under the scheme of Development of RNAi Technology No. PR12894/AGR/36/629/2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhat, H.F., Adams, M.E. & Khanday, F.A. Syntrophin proteins as Santa Claus: role(s) in cell signal transduction. Cell. Mol. Life Sci. 70, 2533–2554 (2013). https://doi.org/10.1007/s00018-012-1233-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-012-1233-9